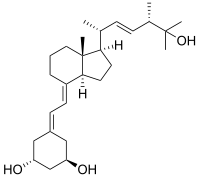
Paricalcitol
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zemplar |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682335 |
| Pregnancy category | |
| Routes of administration | Oral, Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 72%[1] |
| Protein binding | 99.8%[1] |
| Metabolism | Hepatic[1] |
| Biological half-life | 14-20 hours[1] |
| Excretion | Faeces (74%), urine (16%)[1] |
| Identifiers | |
| |
| Synonyms | (1R,3S)-5-[2-[(1R,3aR,7aS)-1-[(2R,5S)-6-hydroxy-5,6-dimethyl-3E-hepten-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-cyclohexane-1,3-diol |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.184.862 |
| Chemical and physical data | |
| Formula | C27H44O3 |
| Molar mass | 416.636 g/mol |
| |
| | |
Paricalcitol (chemically it is 19-nor-1,25-(OH)2-vitamin D2. Marketed by Abbott Laboratories under the trade name Zemplar) is a drug used for the prevention and treatment of secondary hyperparathyroidism (excessive secretion of parathyroid hormone) associated with chronic renal failure. It is an analog of 1,25-dihydroxyergocalciferol, the active form of vitamin D2 (ergocalciferol).
Medical uses
Its primary use in medicine is in the treatment of secondary hyperparathyroidism associated with chronic kidney disease.[2] In three placebo-controlled studies, chronic renal failure patients treated with paricalcitol achieved a mean parathyroid hormone (PTH) reduction of 30% in six weeks. Additionally there was no difference in incidence of hypercalcemia or hyperphosphatemia when compared to placebo.[3] A double-blind randomised study with 263 dialysis patients showed a significant advantage over calcitriol (also known as activated vitamin D3; a similar molecule to 1,25-dihydroxyergocalciferol, adding a methyl group on C24 and lacking a double-bond in the C22 position). After 18 weeks, all patients in the paricalcitol group had reached the target parathormone level of 100 to 300 pg/ml, versus none in the calcitriol group.[4] Combination therapy with paricalcitol and trandolapril has been found to reduce fibrosis in obstructive uropathy.[5] Forty-eight-week therapy with paricalcitol did not alter left ventricular mass index or improve certain measures of diastolic dysfunction in 227 patients with chronic kidney disease.[6]
Adverse effects
Adverse effects by frequency:[1][2][7][8]
Very common (>10% frequency):
- Nausea
Common (1-10% frequency):
- Diarrhoea†
- Oedema
- Allergic reaction
- Arthritis
- Dizziness†
- Stomach discomfort‡
- Gastroesophageal reflux disease†
- Acne†
- Hypercalcaemia†
- Hypocalcaemia†
- Hyperphosphataemia
- Decreased appetite†
- Headache
- Breast tenderness†
- Taste changes
- Hypoparathyroidism
- Vertigo
- Rash‡
Uncommon (0.1-1% frequency):
- Abnormal hepatic enzymes‡
- Constipation‡
- Dry mouth‡
- Itchiness‡
- Hives
- Hypersensitivity‡
- Muscle spasms‡
- Bleeding time prolonged
- Aspartate aminotransferase increased
- Laboratory test abnormal
- Weight loss
- Elevated blood creatinine
- Cardiac arrest
- Arrhythmia
- Atrial flutter
- Anaemia
- Leucopenia
- Lymphadenopathy
- Coma
- Stroke
- Transient ischemic attack
- Fainting
- Myoclonus
- Hypoaesthesia
- Paraesthesia
- Glaucoma
- Conjunctivitis
- Ear disorder
- Pulmonary oedema
- Asthma
- Shortness of breath
- Nose bleed
- Cough
- Rectal haemhorrhage
- Colitis
- Gastritis
- Indigestion
- Difficulty swallowing
- Gastrointestinal disorder
- Gastrointestinal haemorrhage
- Bullous dermatitis
- Hair loss
- Hirsutism
- Hyperhidrosis
- Joint pain
- Joint stiffness
- Back pain
- Muscle twitching
- Muscle aches
- Hyperparathyroidism
- Hyperkalaemia
- Hypocalcemia
- Breast cancer
- Sepsis
- Pneumonia
- Infection
- Pharyngitis
- Vaginal infection
- Influenza
- High blood pressure
- Hypotension
- Gait disturbance
- Injection site pain
- Fever
- Chest pain
- Condition aggravated
- Muscle weakness
- Malaise
- Thirst
- Breast pain
- Impotence
- Confusional state
- Delirium
- Depersonalization
- Agitation
- Insomnia
- Nervousness
‡ These are adverse effects only seen in patients with grade 3 or 4 chronic kidney disease. † These are adverse effects only seen in patients with grade 5 chronic kidney disease.
Contraindications
Contraindications include:[8]
- Vitamin D intoxication
- Hypercalcaemia
- Hypersensitivity to paricalcitol or any of its excipients
whereas cautions include:[1]
- Impaired liver function
- It is also advised that physicians regularly monitor their patients' calcium and phosphorus levels.
Interactions
Drugs that may interact with paricalcitol include:[1][8]
- Ketoconazole, as it may interfere with paricalcitol's metabolism in the liver.
- Digitoxin, hypercalcaemia due to any cause can exacerbate the toxicity of digitoxin.
- Thiazide diuretics or calcium supplements as hypercalcaemia may be induced by this combination
- Magnesium-containing products such as antacids may increase the risk of hypermagnesemia.
- Aluminium-containing products such as antacids may increase the risk of aluminium toxicity.
- Drugs that interfere with the absorption of fat-soluble vitamins, such as cholestyramine may interfere with the absorption of paricalcitol.
Overdose
Electrolyte abnormalities (e.g. hypercalcaemia and hyperphosphataemia) are common overdose symptoms.[8] Treatment is mostly supportive, with particular attention being paid to correcting electrolyte anomalies and reducing intake of calcium in both the form of supplementation and diet.[8] As it is so heavily bound to plasma proteins haemodialysis is unlikely to be helpful in cases of overdose.[8]
Early symptoms of overdose can include:[8]
- Weakness
- Headache
- Somnolence
- Nausea
- Vomiting
- Dry mouth
- Constipation
- Muscle pain
- Bone pain
- Metallic taste in the mouth.
It is worth noting, however, that may of these symptoms are also indicative of kidney failure and hence may be masked by the patient's condition.[8]
Late symptoms of overdose include:
- Loss of appetite
- Weight loss
- Conjunctivitis (calcific)
- Pancreatitis
- Photophobia
- Rhinorrhoea
- Pruritus
- Hyperthermia
- Decreased libido
- Elevated BUN
- Hypercholesterolaemia
- Elevated AST and ALT
- Ectopic calcification
- Hypertension
- Cardiac arrhythmias
- Somnolence
- Death
- Psychosis (rare)
Mechanism of action

Like 1,25-dihydroxyergocalciferol, paricalcitol acts as an agonist at the vitamin D receptor and thereby lowers parathyroid hormone levels in the blood.[1]
Pharmacokinetics
The plasma concentration of paricalcitol decreases rapidly and log-linearly within two hours after initial intravenous administration. Therefore, it is not expected to accumulate with multiple dosing, since paricalcitol is usually given no more frequently than every other day (3 times per week).[9][10]
References
- 1 2 3 4 5 6 7 8 9 "Zemplar (paricalcitol) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 26 January 2014.
- 1 2 Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ↑ "Zemplar: Drug Information"
- ↑ Schubert-Zsilavecz, M, Wurglics, M, Neue Arzneimittel 2005/2006 (in German).
- ↑ Tan, X; He, W; Liu, Y (2009). "Combination therapy with paricalcitol and trandolapril reduces renal fibrosis in obstructive nephropathy". Kidney International. 76 (12): 1248–57. PMID 19759524. doi:10.1038/ki.2009.346.
- ↑ Thadhani, R; Appelbaum, E; Pritchett, Y; Chang, Y; Wenger, J; Tamez, H; Bhan, I; Agarwal, R; et al. (2012). "Vitamin D Therapy and Cardiac Structure and Function in Patients With Chronic Kidney Disease - The PRIMO Randomized Controlled Trial". JAMA. 307 (7): 674–684. PMID 22337679. doi:10.1001/jama.2012.120.
- ↑ "PARICALCITOL capsule, liquid filled [Teva Pharmaceuticals USA Inc]" (PDF). DailyMed. Teva Pharmaceuticals USA Inc. September 2013. Retrieved 26 January 2014.
- 1 2 3 4 5 6 7 8 "Zemplar Soft Capsules 1 mcg - Summary of Product Characteristics". electronic Medicines Compendium. AbbVie Limited. 15 April 2013. Retrieved 26 January 2014.
- ↑ Rxlist: Zemplar
- ↑ Zemplar (paricalcitol) [prescribing information]. North Chicago, IL: AbbVie Inc. October 1, 2015.