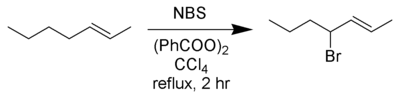
Wohl–Ziegler bromination
| Wohl-Ziegler bromination | |
|---|---|
| Named after | Alfred Wohl Karl Ziegler |
| Reaction type | Substitution reaction |
| Identifiers | |
| Organic Chemistry Portal | wohl-ziegler-reaction |
| RSC ontology ID | RXNO:0000225 |
The Wohl–Ziegler reaction[1][2] is a chemical reaction that involves the allylic or benzylic bromination of hydrocarbons using an N-bromoimide and a radical initiator.[3]
Best yields are achieved with N-bromosuccinimide in carbon tetrachloride solvent. Several reviews have been published.[4][5]
In a typical setup a stoichiometric amount of N-bromosuccinimide solution and a small quantity of initiator are added to a solution of the substrate in CCl4, and the reaction mixture is stirred and heated to the boiling point. Initiation of the reaction is indicated by more vigorous boiling; sometimes the heat source may need to be removed. Once all N-bromosuccinimide (which is denser than the solvent) has been converted to succinimide (which floats on top) the reaction has finished.
References
- ↑ Alfred Wohl (1919). "Bromierung ungesättigter Verbindungen mit N-Brom-acetamid, ein Beitrag zur Lehre vom Verlauf chemischer Vorgänge". Berichte der deutschen chemischen Gesellschaft. 52: 51–63. doi:10.1002/cber.19190520109.
- ↑ Ziegler, K., G. Schenck, E. W. Krockow, A. Siebert, A. Wenz, H. Weber (1942). "Die Synthese des Cantharidins". Justus Liebig's Annalen der Chemie. 551: 1–79. doi:10.1002/jlac.19425510102.
- ↑ Greenwood, F. L.; Kellert, M. D.; Sedlak, J. (1963). "4-Bromo-2-heptene". Org. Synth.; Coll. Vol., 4, p. 108
- ↑ C. Djerassi (1948). "Brominations with N-Bromosuccinimide and Related Compounds. The Wohl–Ziegler Reaction". Chem. Rev. 43 (2): 271–317. PMID 18887958. doi:10.1021/cr60135a004.
- ↑ Horner, L; Winkelman, E. M (1959). "Neuere Methoden der präparativen organischen Chemie II 14. N-Bromsuccinimid, Eigenschaften und Reaktionsweisen Studien zum Ablauf der Substitution XV". Angew. Chem. 71 (11): 349. doi:10.1002/ange.19590711102.