Withanolide

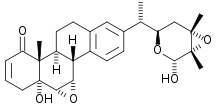

Withanolides are a group of at least 300 naturally occurring steroids built on an ergostane skeleton.[1][2] They occur as secondary metabolites primarily in genera of the Nightshade family, for example in the tomatillo.
Structurally, withanolides consist of a steroid backbone bound to a lactone or one of its derivatives; they are produced via oxidation of steroids. It remains unknown to what end withanolides are produced; they may act as a deterrent for feeding insect larvae and other herbivores.
In the laboratory, many withanolides have exhibited medicinally interesting properties.[3][4]
Genera within the nightshade family that have been found to produce withanolides include: Datura, Dunalia, Iochroma, Lycium, Nicandra, Physalis, Salpichroa, Solanum, Withania, and Jaborosa.
This class of steroidal lactones involves an ergostane-type framework in which C-22 and C-26 are appropriately oxidised to form a (delta)-lactone ring. They are subdivided into nine groups: withanolides, withaphysalins, physalins, nicandrenones, jaborols, ixocarpalactones, perulactones, acnistins and miscellaneous withasteroids.
Examples
Withaferin A, the first withanolide to be isolated, was found in Winter Cherry (Withania somnifera), also known as Ashwagandha in ayurvedic medicine. The anti-inflammatory effects of a few dozen known withanolides have been demonstrated in animal experiments.[5] Withaferin A acts as an anti-angiogenic compound by inhibiting Transcription Factors Sp1 and NF-κB.[6][7] Withaferin A down-regulates angiogenic switch inducer vascular endothelial growth factor (VEGF) gene expression in Ehrlich ascites tumor cells and EAT cells induced angiogenesis on mouse peritoneal cavity.[6] Withaferin A also inhibits angiogenesis on chick chorioallantoic membrane.[6] Withanalides inhibit COX-2 and exhibit anti-inflammatory activity.[8]
Salpichrolides A, B and G (isolated from Salpichroa origanifolia) exhibit an inhibitory effect on the growth of larva of the Mediterranean Fruit Fly (Ceratitis capitata). For this reason, potential pesticide uses for the compounds are being explored.[9]
The nicandrenones of Nicandra physalodes are another group of withanolides with insecticidal effects. 30 years after their discovery, the first total synthesis of nicandrenones was carried out in the year 2000.[10]
Ixocarpalactone A, isolated from the tomatillo (Physalis philadelphica), shows promise as an anti-tumor agent.[11]
References
- ↑ Glotter, E. (1991). "Withanolides and related ergostane-type steroids". Natural Product Reports. 8 (4): 415. ISSN 0265-0568. doi:10.1039/np9910800415.
- ↑ Kirson, Isaac; Glotter, Erwin (1981). "Recent Developments in Naturally Occurring Ergostane-Type Steroids. A Review". Journal of Natural Products. 44 (6): 633–647. ISSN 0163-3864. doi:10.1021/np50018a001.
- ↑ Burton, G.; et al. "Insecticides and feedant deterrants of natural origin related to the withanolides. Isolation, structural elucidation and synthesis". Synthesis, structure and conformation of natural products and analogues of biological importance. University of Buenos Aires.
- ↑ Burton, G.; et al. "Synthesis and structure-activity correlations of steroid hormone analogues". Synthesis, structure and conformation of natural products and analogues of biological importance. University of Buenos Aires.
- ↑ "Ashwagandha". Natural Products. Drugs.com.
- 1 2 3 Prasanna, K. S.; Shilpa, P.; Salimath, B. P. (2009). "Withaferin A suppresses the expression of vascular endothelial growth factor in Ehrlich ascites tumor cells via Sp1 transcription factor" (PDF). Current Trends in Biotechnology and Pharmacy. 3 (2): 138–148.
- ↑ Mohan, R.; Hammers, H. J.; Bargagna-Mohan, P.; Zhan, X. H.; Herbstritt, C. J.; Ruiz, A.; Zhang, L.; Hanson, A. D.; et al. (2004). "Withaferin a is a potent inhibitor of angiogenesis". Angiogenesis. 7 (2): 115–122. PMID 15516832. doi:10.1007/s10456-004-1026-3.
- ↑ Prabhakaran, Y.; Dinakaran, S. K.; Macharala, S. P.; Ghosh, S.; Karanam, S. R.; Kanthasamy, N.; Avasarala, H. (2012). "Molecular docking studies of withanolides against Cox-2 enzyme". Pakistan Journal of Pharmaceutical Sciences. 25 (3): 595–598. PMID 22713947.
- ↑ Bado, S.; Mareggiani, G.; Amiano, N.; Burton, G.; Veleiro, A. S. (2004). "Lethal and Sublethal Effects of Withanolides from Salpichroa origanifolia and Analogues on Ceratitis capitata". Journal of Agricultural and Food Chemistry. 52 (10): 2875–2878. PMID 15137828. doi:10.1021/jf035508a.
- ↑ Stoltz, B. M.; Kano, T.; Corey, E. J. (2000). "Enantioselective Total Synthesis of Nicandrenones". Journal of the American Chemical Society. 122 (37): 9044–9045. doi:10.1021/ja0024892.
- ↑ Kinghorn, A. D.; Su, B. N.; Lee, D.; Gu, J. Q.; Pezzuto, J. M. (2003). "Cancer Chemopreventive Agents Discovered by Activity-Guided Fractionation: An Update". Current Organic Chemistry. 7 (3): 213–226. doi:10.2174/1385272033373003.
