Wiedemann–Franz law
In physics, the Wiedemann–Franz law states that the ratio of the electronic contribution of the thermal conductivity (κ) to the electrical conductivity (σ) of a metal is proportional to the temperature (T).[1]
Theoretically, the proportionality constant L, known as the Lorenz number, is equal to
This empirical law is named after Gustav Wiedemann and Rudolph Franz, who in 1853 reported that κ/σ has approximately the same value for different metals at the same temperature.[2] The proportionality of κ/σ with temperature was discovered by Ludvig Lorenz in 1872.
Qualitatively, this relationship is based upon the fact that the heat and electrical transport both involve the free electrons in the metal.
The mathematical expression of the law can be derived as following. Electrical conduction of metals is a well-known phenomenon and is attributed to the free conduction electrons, which can be measured as sketched in the figure. The current density j is observed to be proportional to the applied electric field and follows Ohm's law where the prefactor is the specific electrical conductivity. Since the electric field and the current density are vectors Ohm's law is expressed here in bold face. The conductivity can in general be expressed as a tensor of the second rank (3×3 matrix). Here we restrict the discussion to isotropic, i.e. scalar conductivity. The specific resistivity is the inverse of the conductivity. Both parameters will be used in the following.
Drude (c. 1900) realized that the phenomenological description of conductivity can be formulated quite generally (electron-, ion-, heat- etc. conductivity). Although the phenomenological description is incorrect for conduction electrons, it can serve as a preliminary treatment.
The assumption is that the electrons move freely in the solid like in an ideal gas. The force applied to the electron by the electric field leads to an acceleration according to
This would lead, however, to a constant acceleration and, ultimately, to an infinite velocity. The further assumption therefore is that the electrons bump into obstacles (like defects or phonons) once in a while which limits their free flight. This establishes an average or drift velocity Vd. The drift velocity is related to the average scattering time as becomes evident from the following relations.
Temperature dependence
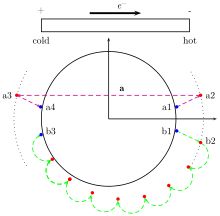
The value L0 = 2.44×10−8 W Ω K−2 results from the fact that at low temperatures ( K) the heat and charge currents are carried by the same quasi-particles: electrons or holes. At finite temperatures two mechanisms produce a deviation of the ratio from the theoretical Lorenz value L0: (i) other thermal carriers such as phonon or magnons, (ii) inelastic scattering. In the 0 temperature limit inelastic scattering is weak and promotes large q scattering values is favored (trajectory a in the figure). For each electron transported a thermal excitation is also carried and the Lorenz number is reached L = L0. Note that in a perfect metal, inelastic scattering would be completely absent in the limit K and the thermal conductivity would vanish . At finite temperature small q scattering values are possible (trajectory b in the figure) and electron can be transported without the transport of an thermal excitation L(T) < L0. In every system at higher temperature the contribution of phonon to thermal transport is important. This can lead to L(T) > L0. Above the Debye temperature the phonon contribution to thermal transport is constant and the ratio L(T) is again found constant.
Limitations of the theory
Experiments have shown that the value of L, while roughly constant, is not exactly the same for all materials. Kittel[5] gives some values of L ranging from L = 2.23×10−8 W Ω K−2 for copper at 0 °C to L = 3.2×10−8 W Ω K−2 for tungsten at 100 °C. Rosenberg[6] notes that the Wiedemann–Franz law is generally valid for high temperatures and for low (i.e., a few Kelvins) temperatures, but may not hold at intermediate temperatures.
In many high purity metals both the electrical and thermal conductivities rise as temperature is decreased. In certain materials (such as silver or aluminum) however, the value of L also may decrease with temperature. In the purest samples of silver and at very low temperatures, L can drop by as much as a factor of 10.[7]
In degenerate semiconductors, the Lorenz number L has a strong dependency on certain system parameters: dimensionality, strength of interatomic interactions and Fermi-level. This law is not valid or the value of the Lorenz number can be reduced at least in following cases: manipulating electronic density of states, varying doping density and layer thickness in superlattices and materials with correlated carriers. [8] [9]
Violations
In 2011, N. Wakeham et al. found that the ratio of the thermal and electrical Hall conductivities in the metallic phase of quasi-one-dimensional Li0.9Mo6O17 diverges with decreasing temperature, reaching a value five orders of magnitude larger than that found in conventional metals obeying the Wiedemann–Franz law.[10][11]
A Berkeley-led study in 2016 by Lee et al. also found a large violation of the Wiedemann–Franz law near the insulator-metal transition in VO2 nanobeams. In the metallic phase, the electronic contribution to thermal conductivity was much smaller than what would be expected from the Wiedemann–Franz law. The results can be explained in terms of independent propagation of charge and heat in a strongly correlated system.[12][13]
See also
References
- ↑ Jones, William; March, Norman H. (1985). Theoretical Solid State Physics. Courier Dover Publications. ISBN 0-486-65016-2.
- ↑ Franz, R.; Wiedemann, G. (1853). "Ueber die Wärme-Leitungsfähigkeit der Metalle". Annalen der Physik (in German). 165 (8): 497–531. Bibcode:1853AnP...165..497F. doi:10.1002/andp.18531650802.
- ↑ Mizutani, Uichiro (2003). Introduction to the Electron Theory of Metals. CAMBRIDGE UNIVERSITY PRESS. ISBN 9780511612626.
- ↑ Thermal conductivity: theory, properties, and applications, edited by Terry Tritt, Kluwer Academic / Plenum Publishers, New York (2004), ISBN 978-0-387-26017-4
- ↑ Kittel, C., 2005. Introduction to Solid State Physics. John Wiley and Sons
- ↑ Rosenberg, H. 2004. The Solid State. Oxford University Press
- ↑ K. Gloos, C. Mitschka, F. Pobell and P. Smeibidl. Cryogenics, 30 (1990), p. 14, doi:10.1016/0011-2275(90)90107-N
- ↑ A. J. Minnich, M. S. Dresselhaus, Z. F. Ren and G. Chen. Bulk nanostructured thermoelectric materials: current research and future prospects, Energy & Environmental Science, 2009, 2, 466–479, doi:10.1039/b822664b
- ↑ Paothep Pichanusakorn, Prabhakar Bandaru. Nanostructured thermoelectrics, Materials Science and Engineering: R: Reports, Volume 67, Issues 2–4, 29 January 2010, pages 19–63, ISSN 0927-796X, doi:10.1016/j.mser.2009.10.001.
- ↑ Wakeham, Nicholas; Bangura, Alimamy F.; Xu, Xiaofeng; Mercure, Jean-Francois; Greenblatt, Martha; Hussey, Nigel E. (2011-07-19). "Gross violation of the Wiedemann–Franz law in a quasi-one-dimensional conductor". Nature Communications. 2. ISSN 2041-1723. PMC 3144592
 . PMID 21772267. doi:10.1038/ncomms1406.
. PMID 21772267. doi:10.1038/ncomms1406. - ↑ "Bristol physicists break 150-year-old law". Retrieved 2017-01-28.
- ↑ Lee, Sangwook; Hippalgaonkar, Kedar; Yang, Fan; Hong, Jiawang; Ko, Changhyun; Suh, Joonki; Liu, Kai; Wang, Kevin; Urban, Jeffrey J. (2017-01-27). "Anomalously low electronic thermal conductivity in metallic vanadium dioxide". Science. 355 (6323): 371–374. ISSN 0036-8075. PMID 28126811. doi:10.1126/science.aag0410.
- ↑ Yang, Sarah (2017-01-26). "For This Metal, Electricity Flows, But Not the Heat | Berkeley Lab". News Center. Retrieved 2017-01-28.
