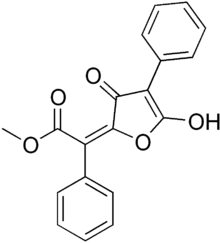
Vulpinic acid
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Methyl (2E)-2-(5-hydroxy-3-oxo-4-phenylfuran-2-ylidene)-2-phenylacetate | |||
| Identifiers | |||
| 3D model (JSmol) |
|||
| ECHA InfoCard | 100.007.560 | ||
| PubChem CID |
|||
| |||
| Properties | |||
| C19H14O5 | |||
| Molar mass | 322.32 g·mol−1 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Vulpinic acid is a naturally occurring methyl ester derivative of pulvinic acid found in several lichen species, as well as some non-lichenized fungi. It was first isolated in 1925.[1] It is bright yellow, and relatively toxic.
Occurrence in lichens

Vulpinic acid was first isolated from lichens, and is a secondary metabolite of the fungal partner. It is speculated that vulpinic acid's biological function is as a repellent for some herbivores. Humans have also exploited this toxicity by using lichens that contain high amounts of the chemical (such as Letharia vulpina) as poison for wolves and foxes.[2][3] The substance showed also some antibacterial activity against gram-positive bacteria and has even been shown to disrupt cell division in MRSA.[4][5] It was also found in Pulveroboletus ravenelii (Berk. & Curt.) Murrill [6][7]
See also
References
- ↑ Mazza, Franc Paolo (1925). "Constitution and physical properties of vulpinic acid". Rend. Accad. Sci. Napoli. 31: 182–90.
- ↑ Lawrey, James D. (1989). "Lichen Secondary Compounds: Evidence for a Correspondence between Antiherbivore and Antimicrobial Function". The Bryologist. The Bryologist, Vol. 92, No. 3. 92 (3): 326–328. JSTOR 3243401. doi:10.2307/3243401.
- ↑ Galun, ed. Margalith (1988). CRC handbook of lichenology. Boca Raton, Fla.: CRC Press. ISBN 0-8493-3583-3.
- ↑ Bačkor, M.; Hudá, J.; Repčák, M.; Ziegler§, W.; Bačkorová, M. (1998). "The Influence of pH and Lichen Metabolites (Vulpinic Acid and (+) Usnic Acid) on the Growth of the Lichen Photobiont Trebouxia Irregularis". The Lichenologist. 30 (6): 577. doi:10.1017/S0024282992000574.
- ↑ Shrestha, Gajendra; Thompson, Andrew; Robison, Richard; St. Clair, Larry L. (28 April 2015). "Letharia vulpina, a vulpinic acid containing lichen, targets cell membrane and cell division processes in methicillin-resistant Staphylococcus aureus". Pharmaceutical Biology. 54 (3): 413–418. doi:10.3109/13880209.2015.1038754.
- ↑ https://edoc.ub.uni-muenchen.de/783/1/Gruber_Gertraud.pdf
- ↑ Gill, M., and Steglich, W. (1987) Pigments of fungi (Macromycetes). Prog Chem Org Nat Prod 51: 1–317.