Von Hippel–Lindau disease
| Von Hippel–Lindau disease | |
|---|---|
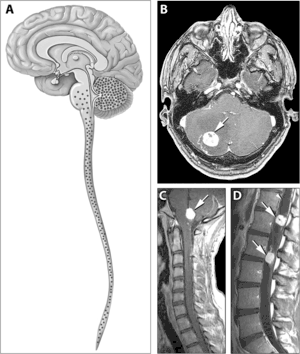 | |
| Typical distribution of hemangioblastomas in von Hippel–Lindau disease. | |
| Classification and external resources | |
| Specialty | medical genetics |
| ICD-10 | Q85.8 |
| ICD-9-CM | 759.6 |
| OMIM | 193300 |
| DiseasesDB | 14000 |
| eMedicine | ped/2417 oph/354 |
| Patient UK | Von Hippel–Lindau disease |
| MeSH | C10.562.400 |
| GeneReviews | |
| Orphanet | 892 |
Von Hippel–Lindau disease (VHL) is a disease which results from a mutation in the von Hippel–Lindau tumor suppressor gene on chromosome 3p25.3.[1][2][3]
Classification
VHL disease can be subdivided according to the clinical manifestations, although these groups often correlate with certain types of mutations present in the VHL gene.[4]
Signs and symptoms
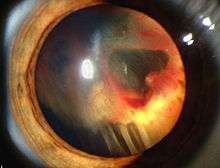
Signs and symptoms associated with VHL disease include headaches, problems with balance and walking, dizziness, weakness of the limbs, vision problems, and high blood pressure. Conditions associated with VHL disease include angiomatosis, hemangioblastomas, pheochromocytoma, renal cell carcinoma, pancreatic cysts (pancreatic serous cystadenoma), endolymphatic sac tumor, and bilateral papillary cystadenomas of the epididymis (men) or broad ligament of the uterus (women).[5][6] Angiomatosis occurs in 37.2% of patients presenting with VHL disease and usually occurs in the retina. As a result, loss of vision is very common. However, other organs can be affected: strokes, heart attacks, and cardiovascular disease are common additional symptoms.[3] Approximately 40% of VHL disease presents with CNS hemangioblastomas and they are present in around 60-80%. Spinal hemangioblastomas are found in 13-59% of VHL disease and are specific because 80% are found in VHL disease.[7][8] Although all of these tumours are common in VHL disease, around half of cases present with only one tumour type.[8]
Pathogenesis
The disease is caused by mutations of the von Hippel–Lindau tumor suppressor (VHL) gene on the short arm of chromosome 3 (3p25-26). There are over 1500 germline mutations and somatic mutations found in VHL disease.[9][10]

Every cell in the body has 2 copies of every gene (bar those found in the sex chromosomes, X and Y). In VHL disease, one copy of the VHL gene has a mutation and produces a faulty VHL protein (pVHL). However, the second copy still produces a functional protein. Tumours form from only those cells where the second copy of the gene has been mutated. A lack of this protein allows tumors characteristic of von Hippel–Lindau syndrome to develop.[11][12]
Approximately 20% of cases of VHL disease are found in individuals without a family history, known as de novo mutations. An inherited mutation of the VHL gene is responsible for the remaining 80 percent of cases.[7]
30-40% of mutations in the VHL gene consist of 50-250kb deletion mutations that remove either part of the gene or the whole gene and flanking regions of DNA. The remaining 60-70% of VHL disease is caused by the truncation of pVHL by nonsense mutations, indel mutations or splice site mutations.[7]
VHL protein

The VHL protein (pVHL) is involved in the regulation of a protein known as hypoxia inducible factor 1α (HIF1α). This is a subunit of a heterodimeric transcription factor that at normal cellular oxygen levels is highly regulated. In normal physiological conditions, pVHL recognizes and binds to HIF1α only when oxygen is present due to the post translational hydroxylation of 2 proline residues within the HIF1α protein. pVHL is an E3 ligase that ubiquitinates HIF1α and causes its degradation by the proteasome. In low oxygen conditions or in cases of VHL disease where the VHL gene is mutated, pVHL does not bind to HIF1α. This allows the subunit to dimerise with HIF1β and activate the transcription of a number of genes, including vascular endothelial growth factor, platelet-derived growth factor B, erythropoietin and genes involved in glucose uptake and metabolism.[12][13] A new novel missense mutation in VHL genes c.194 C>T, c.239 G>A, c.278 G>A, c.319 C>G, c.337 C>G leading to the following variations p.Ala 65 Val, p.Gly 80 Asp, p.Gly 93 Glu, p.Gln 107 Glu, p.Gln 113 Glu in the protein contributed to Renal clear cell carcinoma.[14]
Diagnosis
The detection of tumours specific to VHL disease is important in the disease's diagnosis. In individuals with a family history of VHL disease, one hemangioblastoma, pheochromocytoma or renal cell carcinoma may be sufficient to make a diagnosis. As all the tumours associated with VHL disease can be found sporadically, at least two tumours must be identified to diagnose VHL disease in a person without a family history.[7][8]
Genetic diagnosis is also useful in VHL disease diagnosis. In hereditary VHL, disease techniques such as southern blotting and gene sequencing can be used to analyse DNA and identify mutations. These tests can be used to screen family members of those afflicted with VHL disease; de novo cases that produce genetic mosaicism are more difficult to detect because mutations are not found in the white blood cells that are used for genetic analysis.[7][15]
Treatment
There is no way to reverse VHL mutations, but early recognition and treatment of specific manifestations of VHL can substantially decrease complications and improve quality of life. For this reason, individuals with VHL disease are usually screened routinely for retinal angiomas, CNS hemangioblastomas, clear-cell renal carcinomas and pheochromocytomas.[16] CNS hemangioblastomas are usually surgically removed if they are symptomatic. Photocoagulation and cryotherapy are usually used for the treatment of symptomatic retinal angiomas, although anti-angiogenic treatments may also be an option. Renal tumours may be removed by a partial nephrectomy or other techniques such as radiofrequency ablation.[7]
Epidemiology
VHL disease has an incidence of one in 36,000 births. There is over 90% penetrance by the age of 65.[17] Age at diagnosis varies from infancy to age 60–70 years, with an average patient age at clinical diagnosis of 26 years.
History
The German ophthalmologist Eugen von Hippel first described angiomas in the eye in 1904.[18] Arvid Lindau described the angiomas of the cerebellum and spine in 1927.[19] The term von Hippel-Lindau disease was first used in 1936, however its use became common only in the 1970s.[7]
People
Some descendants of the McCoy family (involved in the Hatfield-McCoy feud of Appalachia, USA) are presumed to have VHL. In an article appearing in the Associated Press, it has been speculated by a Vanderbilt University endocrinologist that the hostility underlying the Hatfield–McCoy feud may have been partly due to the consequences of von Hippel–Lindau disease. The article suggests that the McCoy family was predisposed to bad tempers because many of them had a pheochromocytoma which produced excess adrenaline and a tendency toward explosive tempers.[20]
Nomenclature
Other uncommon names are: angiomatosis retinae, familial cerebello-retinal angiomatosis, cerebelloretinal hemangioblastomatosis, Hippel Disease, Hippel–Lindau syndrome, HLS, VHL, Lindau disease or retinocerebellar angiomatosis.[21][22]
See also
References
- ↑ Richard, S; Gardie, B; Couvé, S; Gad, S (May 30, 2012). "Von Hippel-Lindau: How a rare disease illuminates cancer biology". Seminars in cancer biology. 23 (1): 26–37. PMID 22659535. doi:10.1016/j.semcancer.2012.05.005.
- ↑ Henry, Todd; Campell, James; Hawley, Arthur (1969). Todd-Sanford clinical diagnosis by laboratory methods, edited by Israel Davidsohn [and] John Bernard Henry. (14th ed.). Philadelphia: Saunders. p. 555. ISBN 0-7216-2921-0.
- 1 2 Wong WT; Agrón E; Coleman HR; et al. (February 2007). "Genotype–phenotype correlation in von Hippel–Lindau disease with retinal angiomatosis". Archives of ophthalmology. 125 (2): 239–45. PMC 3019103
 . PMID 17296901. doi:10.1001/archopht.125.2.239. Retrieved 2008-10-22.
. PMID 17296901. doi:10.1001/archopht.125.2.239. Retrieved 2008-10-22. - ↑ Calzada, MJ (March 2010). "Von Hippel-Lindau syndrome: molecular mechanisms of the disease". Clinical & Translational Oncology. 12 (3): 160–5. PMID 20231120. doi:10.1007/s12094-010-0485-9.
- ↑ Lindsay, Kenneth W; Ian Bone; Robin Callander; J. van Gijn (1991). Neurology and Neurosurgery Illustrated. United States: Churchill Livingstone. ISBN 0-443-04345-0.
- ↑ Frantzen, Carlijn; Links, Thera P.; Giles, Rachel H. (21 June 2012). "Von Hippel-Lindau Disease". GeneReviews at NCBI. Retrieved 30 March 2013.
- 1 2 3 4 5 6 7 Maher ER, Glenn GM, Walther M, et al. (June 2011). "von Hippel-Lindau disease: a clinical and scientific review". European Journal of Human Genetics. 19 (6): 617–23. PMC 3110036
 . PMID 21386872. doi:10.1038/ejhg.2010.175.
. PMID 21386872. doi:10.1038/ejhg.2010.175. - 1 2 3 Friedrich, CA (Dec 1, 1999). "Von Hippel-Lindau syndrome. A pleomorphic condition". Cancer. 86 (11 Suppl): 2478–82. PMID 10630173. doi:10.1002/(SICI)1097-0142(19991201)86:11+<2478::AID-CNCR4>3.0.CO;2-5.
- ↑ Kondo, K; Kaelin Jr, WG (10 March 2001). "The von Hippel–Lindau Tumor Suppressor Gene". Experimental Cell Research. 264 (1): 117–125. PMID 11237528. doi:10.1006/excr.2000.5139.
- ↑ Nordstrom-O'Brien M, van der Luijt RB, van Rooijen E, et al. (May 2010). "Genetic analysis of von Hippel-Lindau disease". Hum. Mutat. 31 (5): 521–37. PMID 20151405. doi:10.1002/humu.21219.
- ↑ Knudson, AG (Nov 2001). "Two genetic hits (more or less) to cancer". Nature Reviews Cancer. 1 (2): 157–62. PMID 11905807. doi:10.1038/35101031.
- 1 2 Kaelin, WG (2007). "Von Hippel-Lindau disease". Annual Review of Pathology. 2: 145–73. PMID 18039096. doi:10.1146/annurev.pathol.2.010506.092049.
- ↑ Bader, HL; Hsu, T (Jun 4, 2012). "Systemic VHL gene functions and the VHL disease". FEBS Letters. 586 (11): 1562–9. PMC 3372859
 . PMID 22673568. doi:10.1016/j.febslet.2012.04.032.
. PMID 22673568. doi:10.1016/j.febslet.2012.04.032. - ↑ Pasupuleti Santhosh Kumar, Katari Venkatesh, Lokanathan Srikanth, Potukuchi Venkata Gurunadha Krishna Sarma, Akkamgari Ramprasad Reddy, Srinivasan Subramanian & Bobbidi Venkata Phaneendra (July 2013). "Novel three missense mutations observed in Von Hippel-Lindau gene in a patient reported with renal cell carcinoma". Indian journal of human genetics. 19 (3): 373–376. PMC 3841571
 . PMID 24339559. doi:10.4103/0971-6866.120809.
. PMID 24339559. doi:10.4103/0971-6866.120809. - ↑ Lonser RR (June 2003). "von Hippel-Lindau disease". Lancet. 361 (9374): 2059–67. PMID 12814730. doi:10.1016/S0140-6736(03)13643-4.
- ↑ Priesemann M, Davies KM, Perry LA, et al. (2006). "Benefits of screening in von Hippel-Lindau disease – comparison of morbidity associated with initial tumours in affected parents and children". Horm. Res. 66 (1): 1–5. PMID 16651847. doi:10.1159/000093008.
- ↑ Kim, JJ; Rini, BI; Hansel, DE (2010). "Von Hippel Lindau syndrome". Advances in experimental medicine and biology. Advances in Experimental Medicine and Biology. 685: 228–49. ISBN 978-1-4419-6447-2. PMID 20687511. doi:10.1007/978-1-4419-6448-9_22.
- ↑ Von Hippel E (1904). "Ueber eine sehr seltene Erkrankung der Netzhaut". Albrecht von Graefes Arch Ophthal. 59: 83–106. doi:10.1007/bf01994821.
- ↑ Lindau A (1927). "Zur Frage der Angiomatosis Retinae und Ihrer Hirncomplikation". Acta Ophthalmol. 4: 193–226. doi:10.1111/j.1755-3768.1926.tb07786.x.
- ↑ "Hatfield–McCoy feud blamed on ‘rage’ disease". MSNBC.com. 2007-04-05. Retrieved 2007-04-05.
- ↑ "NORD national organisation for rare disorders".
- ↑ "MeSH (Medical Subject Headings)". Retrieved 08/11/2012. Check date values in:
|access-date=(help)
Kumar PS, Venkatesh K, Srikanth L, Sarma PV, Reddy AR, Subramanian S, Phaneendra BV. Novel three missense mutations observed in Von Hippel-Lindau gene in a patient reported with renal cell carcinoma. Indian J Hum Genet. 2013 Jul;19(3):373-6.
External links
- GeneReviews/NCBI/NIH/UW entry on Von Hippel-Lindau Syndrome
- The VHL Handbook
- Von Hippel–Lindau Disease (VHL) at NINDS
- Von Hippel–Lindau syndrome at NLM Genetics Home Reference
- von Hippel–Lindau syndrome at CHORUS
- Hippel–Lindau disease at Who Named It?
- Online Mendelian Inheritance in Man (OMIM) 608537 (VHL gene)
- VHL Alliance support group
- Cancer.Net: Von Hippel-Lindau Syndrome
- VHLA Newsletter
- VHL Professional Meetings
- VHL Clinical Care Centers