Acute necrotizing ulcerative gingivitis
| Necrotizing ulcerative gingivitis/periodontitis | |
|---|---|
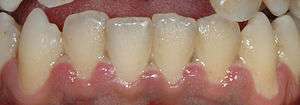 | |
| A fairly mild presentation of acute necrotizing ulcerative gingivitis at the typical site on the gums of the lower front teeth. | |
| Classification and external resources | |
| Specialty | infectious disease |
| ICD-10 | A69.1 |
| ICD-9-CM | 101 |
| DiseasesDB | 13866 |
| MedlinePlus | 001044 |
| MeSH | D005892 |
Acute necrotizing ulcerative gingivitis (ANUG; colloquially known as trench mouth) is a common, non-contagious infection of the gums with sudden onset. The main features are painful, bleeding gums, and ulceration of inter-dental papillae (the sections of gum between adjacent teeth). This disease, along with necrotizing (ulcerative) periodontitis (NP or NUP) is classified as a necrotizing periodontal disease, one of the seven general types of periodontitis. The often severe gingival pain that characterizes ANUG distinguishes it from the more common chronic periodontitis which is rarely painful. ANUG is the acute presentation of necrotizing ulcerative gingivitis (NUG), which is the usual course the disease takes. If improperly treated or neglected, NUG may become chronic and/or recurrent. The causative organisms are mostly anaerobic bacteria, particularly Fusobacteria and spirochete species. Predisposing factors include poor oral hygiene, smoking, malnutrition, psychological stress and immunosuppression (sub-optimal functioning of the immune system). When the attachments of the teeth to the bone are involved, the term NUP is used. Treatment of ANUG is by debridement (although pain may prevent this) and antibiotics (usually metronidazole) in the acute phase, and improving oral hygiene to prevent recurrence. Although the condition has a rapid onset and is debilitating, it usually resolves quickly and does no serious harm. The synonym "trench mouth" arose during World War I as many soldiers developed the disease, probably because of the poor conditions and extreme psychological stress.
Classification
Necrotizing gingivitis is part of a spectrum of disease termed necrotizing periodontal diseases. It is the most minor form of this spectrum, with more advanced stages being termed necrotizing periodontitis, necrotizing stomatitis and the most extreme, cancrum oris. Acute necrotizing ulcerative gingivitis (ANUG) refers to the clinical onset of NUG. The word acute is used because usually the onset is sudden.[1] Other forms of NUG may be chronic or recurrent.
Necrotizing ulcerative periodontitis (NUP) is where the infection leads to attachment loss, and involves only the gingiva, periodontal ligament and alveolar ligament.[1][2][3] Progression of the disease into tissue beyond the mucogingival junction characterizises necrotizing stomatitis.
Signs and symptoms
In the early stages some patients may complain of a feeling of tightness around the teeth.[1] Three signs/symptoms must be present to diagnose this condition:[1]
- Severe gingival pain.[4]
- Profuse gingival bleeding that requires little or no provocation.[1]
- Interdental papillae are ulcerated with necrotic slough.[4] The papillary necrosis of NUG has been described as "punched out".[1]
Other signs and symptoms may be present, but not always.[1]
Malaise, fever and/or cervical lymph node enlargement are rare (unlike the typical features of herpetic stomatitis).[4] Pain is fairly well localized to the affected areas.[4] Systemic reactions may be more pronounced in children.[1] Cancrum oris (noma) is a very rare complication, usually in debilitated children.[4] Similar features but with more intense pain may be seen in necrotizing periodontitis in HIV/AIDS.[4]
Causes
Necrotizing periodontal disease is caused by a mixed bacterial infection that includes anaerobes such as P. intermedia[3] and Fusobacterium as well as spirochetes, such as Treponema.[4]
ANUG may also be associated with diseases in which the immune system is compromised, including HIV/AIDS.[2] ANUG is an opportunistic infection that occurs on a background of impaired local or systemic host defenses. The predisposing factors for ANUG are smoking, psychological stress, malnutrition and immunosuppression.
Zones of infection have been described. These are (superficial to deep) the bacterial zone, the neutrophil rich zone, the necrotic zone and the spirochetal zone.
Diagnosis
Diagnosis is usually clinical.[4] Smear for fusospirochaetal bacteria and leukocytes; blood picture occasionally.[4] The important differentiation is with acute leukaemia or herpetic stomatitis.[4]
Treatment
Treatment includes irrigation and debridement of necrotic areas (areas of dead and/or dying gum tissue), oral hygiene instruction and the uses of mouth rinses and pain medication. If there is systemic involvement, then oral antibiotics may be given, such as metronidazole.[4] As these diseases are often associated with systemic medical issues, proper management of the systemic disorders is appropriate.[2]
Prognosis
Untreated, the infection may lead to rapid destruction of the periodontium and can spread, as necrotizing stomatitis or noma, into neighbouring tissues in the cheeks, lips or the bones of the jaw. As stated, the condition can occur and be especially dangerous in people with weakened immune systems. This progression to noma is possible in malnourished susceptible individuals, with severe disfigurement possible.
Epidemiology
In developed countries, this disease occurs mostly in young adults. In developing countries, NUG may occur in children of low socioeconomic status, usually occurring with malnutrition (especially inadequate protein intake) and shortly after the onset of viral infections (e.g. measles).[1]
Predisposing factors include smoking, viral respiratory infections and immune defects, such as in HIV/AIDS. Uncommon, except in lower socioeconomic classes, this typically affects adolescents and young adults, especially in institutions, armed forces, etc., or people with HIV/AIDS.[4] The disease has occurred in epidemic-like patterns, but it is not contagious.[1]
History
Necrotizing ulcerative gingivitis has been observed for centuries. Xenophon observes sore mouth and foul smelling breath in Greek soldiers in the 4th century BC. Hunter describes the clinical features of ANUG in 1778, differentiating it from scurvy (avitaminosis C) and chronic periodontitis. Jean Hyacinthe Vincent, a French physician working at the Paris Pasteur Institute describes a fusospirochetal infection of the pharynx and palatine tonsils, causing "ulcero-membranous pharyngitis and tonsillitis",[5] which later became known as Vincent's angina. Later in 1904, Vincent describes the same pathogenic organisms in "ulceronecrotic gingivitis". Vincent's angina is sometimes confused with NUG, however the former is tonsillitis and pharyngitis, and the latter involves the gums, and usually the two conditions occur in isolation from each other.
The term trench mouth evolved because the disease was observed in front line soldiers during World War I, thought to be a result at least partly because of extreme psychologic stress they were exposed to.[1] The same condition was appearing in civilians during periods of bombing raids, who were away from the front line, and who had relatively good diets during wartime due to rationing, so it is assumed that psychologic stress was the significant causative factor. It has also been associated with high tobacco use in the army.
Many other historical names for this condition (and Vincent's angina) have occurred, including: "acute membranous gingivitis", "fusospirillary gingivitis", " fusospirillosis", "fusospirochetal gingivitis", "phagedenic gingivitis", "Vincent stomatitis", "Vincent gingivitis", and "Vincent infection".[6]
In the late 1980s-early 1990s, it was originally thought that some necrotizing periodontal diseases seen in severely affected aids patients were strictly a sequela of HIV, and it was even called HIV-associated periodontitis.[7] It is now understood that its association with HIV/AIDS was due to the immunocompromised status of such patients, and it also occurs with higher prevalence in association with other diseases in which the immune system is compromised.[2]
References
- 1 2 3 4 5 6 7 8 9 10 11 Karring, edited by Jan Lindhe, Niklaus P. Lang, Thorkild (2008). Clinical periodontology and implant dentistry (5th ed.). Oxford: Blackwell Munksgaard. pp. 413, 459. ISBN 9781405160995.
- 1 2 3 4 American Academy of Periodontology (May 2000). "Parameter on acute periodontal diseases. American Academy of Periodontology" (PDF). J. Periodontol. 71 (5 Suppl): 863–6. PMID 10875694. doi:10.1902/jop.2000.71.5-S.863. Archived from the original (PDF) on 2010-11-28.
- 1 2 American Academy of Periodontology (1999). "Consensus report: Necrotizing Periodontal Diseases". Ann. Periodontol. 4 (1): 78. doi:10.1902/annals.1999.4.1.78.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Scully, Crispian (2008). Oral and maxillofacial medicine : the basis of diagnosis and treatment (2nd ed.). Edinburgh: Churchill Livingstone. pp. 101, 347. ISBN 9780443068188.
- ↑ Taylor, FE; McKinstry, WH (1917). "The Relation of Peri-dental Gingivitis to Vincent's Angina." (PDF). Proceedings of the Royal Society of Medicine. 10 (Laryngol Sect): 43–8. PMC 2017821
 . PMID 19979715.
. PMID 19979715. - ↑ "Definition of Vincent angina". Medterms.com. 2001-09-13. Retrieved 2010-02-13.
- ↑ NYS Department of Health AIDS Institute. "Clinical Manifestations and Management of HIV-Related Periodontal Disease". Oral Health Care for People with HIV Infection: HIV Clinical Guidelines. p. 31.