Venus flytrap
| Venus flytrap | |
|---|---|
 | |
| Leaf | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Order: | Caryophyllales |
| Family: | Droseraceae |
| Genus: | Dionaea |
| Species: | D. muscipula |
| Binomial name | |
| Dionaea muscipula Sol. ex J.Ellis 1768 | |
.svg.png) | |
| Distribution | |
| Synonyms[2] | |
| |
The Venus flytrap (also referred to as Venus's flytrap or Venus' flytrap), Dionaea muscipula, is a carnivorous plant native to subtropical wetlands on the East Coast of the United States in North Carolina and South Carolina.[3] It catches its prey—chiefly insects and arachnids—with a trapping structure formed by the terminal portion of each of the plant's leaves, which is triggered by tiny hairs on their inner surfaces. When an insect or spider crawling along the leaves contacts a hair, the trap prepares to close, snapping shut only if another contact occurs within approximately twenty seconds of the first strike. The requirement of redundant triggering in this mechanism serves as a safeguard against wasting energy by trapping objects with no nutritional value, and the plant will only begin digestion after five more stimuli to ensure it has caught a live bug worthy of consumption.
Dionaea is a monotypic genus closely related to the waterwheel plant (Aldrovanda vesiculosa) and sundews (Drosera), all of which belong to the family Droseraceae.
Description
The Venus flytrap is a small plant whose structure can be described as a rosette of four to seven leaves, which arise from a short subterranean stem that is actually a bulb-like object. Each stem reaches a maximum size of about three to ten centimeters, depending on the time of year;[4] longer leaves with robust traps are usually formed after flowering. Flytraps that have more than seven leaves are colonies formed by rosettes that have divided beneath the ground.

The leaf blade is divided into two regions: a flat, heart-shaped photosynthesis-capable petiole, and a pair of terminal lobes hinged at the midrib, forming the trap which is the true leaf. The upper surface of these lobes contains red anthocyanin pigments and its edges secrete mucilage. The lobes exhibit rapid plant movements, snapping shut when stimulated by prey. The trapping mechanism is tripped when prey contacts one of the three hair-like trichomes that are found on the upper surface of each of the lobes. The mechanism is so highly specialized that it can distinguish between living prey and non-prey stimuli, such as falling raindrops;[5] two trigger hairs must be touched in succession within 20 seconds of each other or one hair touched twice in rapid succession,[5] whereupon the lobes of the trap will snap shut, typically in about one-tenth of a second.[6] The edges of the lobes are fringed by stiff hair-like protrusions or cilia, which mesh together and prevent large prey from escaping. These protrusions, and the trigger hairs (also known as sensitive hairs) are likely homologous with the tentacles found in this plant’s close relatives, the sundews. Scientists have concluded that the snap trap evolved from a fly-paper trap similar to that of Drosera.[7]
The holes in the meshwork allow small prey to escape, presumably because the benefit that would be obtained from them would be less than the cost of digesting them. If the prey is too small and escapes, the trap will usually reopen within 12 hours. If the prey moves around in the trap, it tightens and digestion begins more quickly.
Speed of closing can vary depending on the amount of humidity, light, size of prey, and general growing conditions. The speed with which traps close can be used as an indicator of a plant's general health. Venus flytraps are not as humidity-dependent as are some other carnivorous plants, such as Nepenthes, Cephalotus, most Heliamphora, and some Drosera.
The Venus flytrap exhibits variations in petiole shape and length and whether the leaf lies flat on the ground or extends up at an angle of about 40–60 degrees. The four major forms are: 'typica', the most common, with broad decumbent petioles; 'erecta', with leaves at a 45-degree angle; 'linearis', with narrow petioles and leaves at 45 degrees; and 'filiformis', with extremely narrow or linear petioles. Except for 'filiformis', all of these can be stages in leaf production of any plant depending on season (decumbent in summer versus short versus semi-erect in spring), length of photoperiod (long petioles in spring versus short in summer), and intensity of light (wide petioles in low light intensity versus narrow in brighter light).
|
Etymology
The plant's common name refers to Venus, the Roman goddess of love. The genus name, Dionaea ("daughter of Dione"), refers to the Greek goddess Aphrodite, while the species name, muscipula, is Latin for "mousetrap".[8]
Historically, the plant was also known by the slang term "tipitiwitchet" or "tippity twitchet", possibly an oblique reference to the plant's resemblance to human female genitalia.[8][9]
Carnivory

Prey selectivity
Most carnivorous plants selectively feed on specific prey. This selection is due to the available prey and the type of trap used by the organism. With the Venus flytrap, prey is limited to beetles, spiders and other crawling arthropods. In fact, the Dionaea diet is 33% ants, 30% spiders, 10% beetles, and 10% grasshoppers, with fewer than 5% flying insects.[10] Given that Dionaea evolved from an ancestral form of Drosera (carnivorous plants that use a sticky trap instead of a snap trap) the reason for this evolutionary branching becomes clear. Whilst Drosera consume smaller, aerial insects, Dionaea consume larger terrestrial bugs. Dionaea are able to extract more nutrients from these larger bugs. This gives Dionaea an evolutionary advantage over their ancestral sticky trap form.[11]
Mechanism of trapping

The Venus flytrap is one of a very small group of plants capable of rapid movement, such as Mimosa pudica, the Telegraph plant, sundews and bladderworts.
The mechanism by which the trap snaps shut involves a complex interaction between elasticity, turgor and growth. The trap only shuts when there have been two stimulations of the trigger hairs; this is to avoid inadvertent triggering of the mechanism by dust and other wind-borne debris. In the open, untripped state, the lobes are convex (bent outwards), but in the closed state, the lobes are concave (forming a cavity). It is the rapid flipping of this bistable state that closes the trap,[6] but the mechanism by which this occurs is still poorly understood. When the trigger hairs are stimulated, an action potential (mostly involving calcium ions—see calcium in biology) is generated, which propagates across the lobes and stimulates cells in the lobes and in the midrib between them.[12] It is hypothesized that there is a threshold of ion buildup for the Venus flytrap to react to stimulation.[13] After closing, the flytrap counts additional stimulations of the trigger hairs, to five total, to start the production of digesting enzymes.[14] The acid growth theory states that individual cells in the outer layers of the lobes and midrib rapidly move 1H+ (hydrogen ions) into their cell walls, lowering the pH and loosening the extracellular components, which allows them to swell rapidly by osmosis, thus elongating and changing the shape of the trap lobe. Alternatively, cells in the inner layers of the lobes and midrib may rapidly secrete other ions, allowing water to follow by osmosis, and the cells to collapse. Both of these mechanisms may play a role and have some experimental evidence to support them.[15][16]
Digestion
If the prey is unable to escape, it will continue to stimulate the inner surface of the lobes, and this causes a further growth response that forces the edges of the lobes together, eventually sealing the trap hermetically and forming a "stomach" in which digestion occurs. Release of the digestive enzymes is controlled by the hormone jasmonic acid, the same hormone that triggers the release of toxins as an anti-herbivore defense mechanism in non-carnivorous plants. (See Evolution below)[14][17] Once the digestive glands in the leaf lobes have been activated, digestion is catalysed by hydrolase enzymes secreted by the glands.
Oxidative protein modification is likely to be a pre-digestive mechanism used by Dionaea muscipula. Aqueous leaf extracts have been found to contain quinones such as the naphthoquinone plumbagin that couples to different NADH-dependent diaphorases to produce superoxide and hydrogen peroxide upon autoxidation.[18] Such oxidative modification could rupture animal cell membranes. Plumbagin is known to induce apoptosis, associated with the regulation of the Bcl-2 family of proteins.[19] When the Dionaea extracts were pre-incubated with diaphorases and NADH in the presence of serum albumin (SA), subsequent tryptic digestion of SA was facilitated.[18] Since the secretory glands of Droseraceae contain proteases and possibly other degradative enzymes, it may be that the presence of oxygen-activating redox cofactors function as extracellular pre-digestive oxidants to render membrane-bound proteins of the prey (insects) more susceptible to proteolytic attacks.[18]
Digestion takes about ten days, after which the prey is reduced to a husk of chitin. The trap then reopens, and is ready for reuse.[20]
Evolution

Carnivory in plants is a very specialized form of foliar feeding, and is an adaptation found in several plants that grow in nutrient-poor soil. Carnivorous traps were naturally selected to allow these organisms to compensate for the nutrient deficiencies of their harsh environments by supplementing ordinary photosynthate with animal proteins.[21]
The "snap trap" mechanism characteristic of Dionaea is shared with only one other carnivorous plant genus, Aldrovanda. For most of the 20th century, this relationship was thought to be coincidental, more precisely an example of convergent evolution. Some phylogenetic studies even suggested that the closest living relatives of Aldrovanda were the sundews.[22] It was not until 2002 that a molecular evolutionary study, by analyzing combined nuclear and chloroplast DNA sequences, indicated that Dionaea and Aldrovanda were closely related and that the snap trap mechanism evolved only once in a common ancestor of the two genera.[23][24]
A 2009 study[22] presented evidence for the evolution of snap traps of Dionaea and Aldrovanda from a flypaper trap like Drosera regia, based on molecular data. The molecular and physiological data imply that Dionaea and Aldrovanda snap traps evolved from the flypaper traps of a common ancestor with Drosera. Pre-adaptations to the evolution of snap traps were identified in several species of Drosera, such as rapid leaf and tentacle movement. The model proposes that plant carnivory by snap trap evolved from the flypaper traps, driven by increasing prey size. Bigger prey provides greater nutritional value, but large insects can easily escape the sticky mucilage of flypaper traps; the evolution of snap traps would therefore prevent escape and kleptoparasitism (theft of prey captured by the plant before it can derive benefit from it), and would also permit a more complete digestion.[22][23]
In 2016, a study of the expression of genes in the plant's leaves as they captured and digested prey was published in the journal, Genome Research. The gene activation observed in the leaves of the plants gives support to the hypothesis that the carnivorous mechanisms present in the flytrap are a specially adapted version of mechanisms used by non-carnivorous plants to defend against herbivorous insects.[17][25] In many non-carnivorous plants, jasmonic acid serves as a signaling molecule for the activation of defense mechanisms, such as the production of hydrolases, which can destroy chitin and other molecular components of insect and microbial pests.[26] In the Venus flytrap, this same molecule has been found to be responsible for the activation of the plant's digestive glands. A few hours after the capture of prey, another set of genes is activated inside the glands, the same set of genes that is active in the roots of other plants, allowing them to absorb nutrients. The use of similar biological pathways in the traps as non-carnivorous plants use for other purposes indicates that somewhere in its evolutionary history, the Venus flytrap repurposed these genes for the purpose of carnivory.
Proposed evolutionary history
Carnivorous plants are generally herbs, and their traps the result of primary growth. They generally do not form readily fossilizable structures such as thick bark or wood. As a result, there is no fossil evidence of the steps that might link Dionaea and Aldrovanda, or either genus with their common ancestor, Drosera. Nevertheless, it is possible to infer an evolutionary history based on phylogenetic studies of both genera. Researchers have proposed a series of steps that would ultimately result in the complex snap-trap mechanism:[22][23]
- Larger insects usually walk over the plant, instead of flying to it,[27] and are more likely to break free from sticky glands alone. Therefore, a plant with wider leaves, like Drosera falconeri,[22] must have adapted to move the trap and its stalks in directions that maximized its chance of capturing and retaining such prey—in this particular case, longitudinally. Once adequately "wrapped", escape would be more difficult.[27]
- Evolutionary pressure then selected for plants with shorter response time, in a manner similar to Drosera burmannii or Drosera glanduligera. The faster the closing, the less reliant on the flypaper model the plant would be.
- As the trap became more and more active, the energy required to "wrap" the prey increased. Plants that could somehow differentiate between actual insects and random detritus/rain droplets would have an advantage, thus explaining the specialization of inner tentacles into trigger hairs.
- Ultimately, as the plant relied more on closing around the insect rather than gluing them to the leaf surface, the tentacles so evident in Drosera would lose their original function altogether, becoming the "teeth" and trigger hairs—an example of natural selection utilizing pre-existing structures for new functions.
- Completing the transition, the plant eventually developed the depressed digestive glands found inside the trap, rather than using the dews in the stalks, further differentiating it from genus Drosera.
Habitat
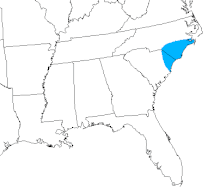
The Venus flytrap is found in nitrogen- and phosphorus-poor environments, such as bogs and wet savannahs. Small in stature and slow-growing, the Venus flytrap tolerates fire well, and depends on periodic burning to suppress its competition.[28] Fire suppression threatens its future in the wild.[29] It survives in wet sandy and peaty soils. Although it has been successfully transplanted and grown in many locales around the world, it is native only to the coastal bogs of North and South Carolina in the United States, specifically within a 60-mile radius of Wilmington, North Carolina.[30] One such place is North Carolina's Green Swamp. There also appears to be a naturalized population of Venus flytraps in northern Florida as well as an introduced population in western Washington.[31][32] The nutritional poverty of the soil is the reason that the plant relies on such elaborate traps: insect prey provide the nitrogen for protein formation that the soil cannot. The Venus flytrap is not a tropical plant and can tolerate mild winters. In fact, Venus flytraps that do not go through a period of winter dormancy will weaken and die after a period of time.[33]
Cultivation

Venus flytraps are popular as cultivated plants, but have a reputation for being difficult to grow.[4] Successfully growing these specialized plants requires recreating a close approximation to the plant's natural habitat.
Healthy Venus flytraps will produce scapes of white flowers in spring; however, many growers remove the flowering stems early (2–3 inches), as flowering consumes some of the plant's energy and thereby reduces the rate of trap production. If healthy plants are allowed to flower, successful pollination will result in seeds.
Plants can be propagated by seed, taking around four to five years to reach maturity. More commonly, they are propagated by clonal division in spring or summer. Venus flytraps can also be propagated in vitro using plant tissue culture.[34] Most Venus flytraps found for sale in nurseries garden centers have been produced using this method, as this is the most cost-effective way to propagate them on a large scale. Regardless of the propagation method used, the plants will live for 20 to 30 years if cultivated in the right conditions.[35]
Cultivars
Venus flytraps are by far the most commonly recognized and cultivated carnivorous plant, and they are frequently sold as houseplants. Various cultivars (cultivated varieties) have come into the market through tissue culture of selected genetic mutations, and these plants are raised in large quantities for commercial markets.
Conservation
The species is classified as "vulnerable" by the National Wildlife Federation.[36] In 2015, there were estimated to be fewer than 33,000 plants in the wild, all within 75 miles (121 km) of the city of Wilmington, North Carolina, and all on sites owned by The Nature Conservancy, the North Carolina state government, or the US military.[37]
In 2014, the state of North Carolina passed Senate Bill 734 which classifies the theft of naturally growing Venus flytraps in the state as a felony. Tougher sanctions and penalties for the theft were also enacted in December 1, 2014 in accordance with legislation.[38]
In alternative medicine
Venus flytrap extract is available on the market as an herbal remedy, sometimes as the prime ingredient of a patent medicine named "Carnivora". According to the American Cancer Society, these products are promoted in alternative medicine as a treatment for a variety of human ailments including HIV, Crohn's disease and skin cancer, but "available scientific evidence does not support the health claims made for Venus flytrap extract".[39]
See also
References
- ↑ Schnell, D.; Catling, P.; Folkerts, G.; Frost, C.; Gardner, R.; et al. (2000). "Dionaea muscipula". IUCN Red List of Threatened Species. Version 2006. International Union for Conservation of Nature. Retrieved 11 May 2006. Listed as Vulnerable (VU A1acd, B1+2c v2.3)
- ↑ Schlauer, J. (N.d.) Dionaea muscipula. Carnivorous Plant Database.
- ↑ Kew World Checklist of Selected Plant Families
- 1 2 "Venus flytraps". The Carnivorous Plant FAQ. Retrieved 2005-06-13.
- 1 2 Raven, Peter H.; Evert, Ray Franklin; Eichhorn, Susan E. (2005). Biology of Plants (7th ed.). W.H. Freeman and Company. ISBN 0-7167-1007-2.
- 1 2 Forterre, Yoël; Skotheim, Jan M.; Dumais, Jacques; Mahadevan, L. (27 January 2005). "How the Venus flytrap snaps" (PDF). Nature. 433 (7024): 421–425. PMID 15674293. doi:10.1038/nature03185. Archived from the original (PDF) on 2 December 2007.
- ↑ Cameron, Kenneth M.; Wurdack, Kenneth J.; Jobson, Richard W. (2002). "Molecular evidence for the common origin of snap-traps among carnivorous plants". American Journal of Botany. 89 (9): 1503–1509. PMID 21665752. doi:10.3732/ajb.89.9.1503.
- 1 2 "Background Information on Venus Fly Traps—Venus Fly Trap naming and history". FlyTrapCare.com. 2008-04-04. Archived from the original on 17 December 2008.
- ↑ Rice, Barry (January 2007). "How did the Venus flytrap get its name?". The Carnivorous Plant FAQ.
- ↑ Ellison, DM; Gotelli, NJ (2009). "Energetics and the evolution of carnivorous plants—Darwin's 'Most Wonderful plants in the world'" (PDF). Journal of Experimental Botany. 60 (1): 19–42. PMID 19213724. doi:10.1093/jxb/ern179.
- ↑ Gibson, TC; Waller, DM (2009). "Evolving Darwin's 'most wonderful' plant: ecological steps to a snap-trap". New Phytologist. 183 (1): 575–587. PMID 19573135. doi:10.1111/j.1469-8137.2009.02935.x.
- ↑ Hodick, Dieter; Sievers, Andreas (1989). "The action potential of Dionaea muscipula Ellis". Planta. 174 (1): 8–18. doi:10.1007/BF00394867.
- ↑ "Press release: The Trap Snaps Shut" (PDF). Wiley ChemBioChem. January 2010. doi:10.1002/(issn)1439-7633/asset/homepages/press/201001press.pdf.
- 1 2 Böhm, J.; Scherzer, S.; Krol, E.; Kreuzer, I.; von Meyer, K.; Lorey, C.; Mueller, T. D.; Shabala, L.; Monte, I.; Solano, R.; Al-Rasheid, K. A. S.; Rennenberg, H.; Shabala, S.; Neher, E.; Hedrich, R. (2016). "The Venus Flytrap Dionaea muscipula Counts Prey-Induced Action Potentials to Induce Sodium Uptake". Current Biology. 26: 286–295. ISSN 0960-9822. PMC 4751343
 . PMID 26804557. doi:10.1016/j.cub.2015.11.057.
. PMID 26804557. doi:10.1016/j.cub.2015.11.057. - ↑ Williams, S. E. 2002. Comparative physiology of the Droseraceae sensu stricto—How do tentacles bend and traps close? Proceedings of the 4th International Carnivorous Plant Society Conference. Tokyo, Japan. pp. 77–81.
- ↑ Hodick, Dieter; Sievers, Andreas (1988). "On the mechanism of closure of Venus flytrap (Dionaea muscipula Ellis)". Planta. 179 (1): 32–42. PMID 24201419. doi:10.1007/BF00395768.
- 1 2 Bemm, Felix; Becker, Dirk; Larisch, Christina; Kreuzer, Ines; Escalante-Perez, Maria; Schulze, Waltraud X.; Ankenbrand, Markus; Weyer, Anna-Lena Van de; Krol, Elzbieta; Al-Rasheid, Khaled A.; Mithöfer, Axel; Weber, Andreas P.; Schultz, Jörg; Hedrich, Rainer (4 May 2016). "Venus flytrap carnivorous lifestyle builds on herbivore defense strategies". Genome Research. 26: 812–825. ISSN 1088-9051. PMC 4889972
 . PMID 27197216. doi:10.1101/gr.202200.115. Retrieved 26 May 2016.
. PMID 27197216. doi:10.1101/gr.202200.115. Retrieved 26 May 2016. - 1 2 3 Galek H, Osswald WF, Elstner EF (1990). "Oxidative protein modification as predigestive mechanism of the carnivorous plant Dionaea muscipula: an hypothesis based on in vitro experiments". Free Radic Biol Med. 9 (5): 427–34. PMID 2292436. doi:10.1016/0891-5849(90)90020-J.
- ↑ Hsu YL, Cho CY, Kuo PL, Huang YT, Lin CC (Aug 2006). "Plumbagin (5-Hydroxy-2-methyl-1,4-naphthoquinone) Induces Apoptosis and Cell Cycle Arrest in A549 Cells through p53 Accumulation via c-Jun NH2-Terminal Kinase-Mediated Phosphorylation at Serine 15 in Vitro and in Vivo". J Pharmacol Exp Ther. 318 (2): 484–94. PMID 16632641. doi:10.1124/jpet.105.098863.
- ↑ Produced by Neil Lucas (2009-12-07). "Plants". Life. BBC. BBC One.
- ↑ AM Ellison (2006). "Nutrient limitation and stoichiometry of carnivorous plants" (PDF). Biology. 8: 740–747. doi:10.1055/s-2006-923956.
- 1 2 3 4 5 Gibson, T. C.; Waller, D. M. (2009). "Evolving Darwin's 'most wonderful' plant: ecological steps to a snap-trap" (PDF). New Phytologist. 183 (3): 575–587. PMID 19573135. doi:10.1111/j.1469-8137.2009.02935.x.
- 1 2 3 Cameron, K. M.; Wurdack, K. J.; Jobson, R. W. (2002). "Molecular evidence for the common origin of snap-traps among carnivorous plants". American Journal of Botany. 89 (9): 1503–1509. PMID 21665752. doi:10.3732/ajb.89.9.1503.
- ↑ Rivadavia, F.; K. Kondo; M. Kato & M. Hasebe (2003). "Phylogeny of the sundews, Drosera (Droseraceae), based on chloroplast rbcL and nuclear 18S ribosomal DNA Sequences". American Journal of Botany. 90 (1): 123–130. PMID 21659087. doi:10.3732/ajb.90.1.123.
- ↑ Stokstad, E. (12 May 2016). "How the Venus flytrap acquired its taste for meat". Science. 352 (6287): 756–756. PMID 27174967. doi:10.1126/science.352.6287.756. Retrieved 26 May 2016.
- ↑ Turner, John G.; Ellis, Christine; Devoto, Alessandra (1 May 2002). "The Jasmonate Signal Pathway". The Plant Cell. 14 (suppl 1): S153–S164. ISSN 1532-298X. PMC 151253
 . PMID 12045275. doi:10.1105/tpc.000679. Retrieved 26 May 2016.
. PMID 12045275. doi:10.1105/tpc.000679. Retrieved 26 May 2016. - 1 2 "Venus flytrap origins uncovered". BBC News. 2009.
- ↑ W. Schulze; E.D. Schulze; I. Schulze & R. Oren (2001). "Quantification of insect nitrogen utilization by the venus fly trap Dionaea muscipula catching prey with highly variable isotope signatures". Journal of Experimental Botany. 52 (358): 1041–1049. PMID 11432920. doi:10.1093/jexbot/52.358.1041.
- ↑ Leege, Lissa. "How does the Venus flytrap digest flies?". Scientific American. Retrieved 2008-08-20.
- ↑ Darwin, C. R. 1875. Insectivorous Plants.
- ↑ Schnell, D. E. (2002). Carnivorous Plants of the United States and Canada (2nd ed.). Timber Press. ISBN 0-88192-540-3.
- ↑ Giblin, D. Nd. Dionaea muscipula. Burke Museum of Natural History and Culture.
- ↑ "International Carnivorous Plant Society". Carnivorousplants.org. Archived from the original on 28 July 2014. Retrieved 2013-08-26.
- ↑ Jang, Gi-Won; Kim, Kwang-Soo; Park, Ro-Dong (2003). "Micropropagation of Venus fly trap by shoot culture". Plant Cell, Tissue and Organ Culture. 72 (1): 95–98. doi:10.1023/A:1021203811457. Retrieved 19 May 2016.
- ↑ D'Amato, Peter (1998). The Savage Garden: Cultivating Carnivorous Plants. Berkeley, California: Ten Speed Press. ISBN 0-89815-915-6.
- ↑ "Venus Flytrap". National Wildlife Federation. Retrieved 2015-07-10.
- ↑ John R. Platt (2015-01-22). "Venus Flytraps Risk Extinction in the Wild at the Hands of Poachers". Scientific American. Retrieved 2015-07-10.
- ↑ Evans, Jon (September 18, 2014). "Stealing Venus Flytrap plants now a felony". www.wect.com. Retrieved July 21, 2017.
- ↑ "Venus Flytrap". American Cancer Society. November 2008. Archived from the original on 18 February 2015. Retrieved 22 September 2013.
External links
| Wikispecies has information related to: Dionaea muscipula |
| Wikimedia Commons has media related to Dionaea muscipula. |
- Images and movies of the Venus flytrap (Dionaea muscipula) at ARKive
- Venus Flytrap Growing Guide and Distribution Map
- The Carnivorous Plant FAQ: Flytraps
- Venus flytrap origins uncovered - BBC
- The CP Photo Finder: "Dionaea"
- Dionaea muscipula - The Venus flytrap at Botanical Society of America
- Criminal Podcast Episode Five: Dropping Like Flies



