Ventrolateral preoptic nucleus
| Ventrolateral Preoptic Nucleus | |
|---|---|
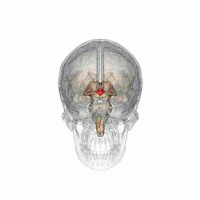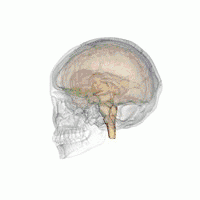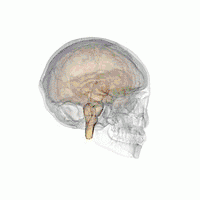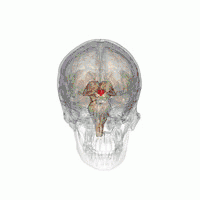 The VLPO is located at the anterior of the Hypothalamus. It is also called the intermediate nucleus of the preoptic area | |
| Details | |
| Part of | Preoptic nucleus |
| Identifiers | |
| Acronym(s) | VLPO or IPA |
| NeuroNames | hier-3122 |
The ventrolateral preoptic nucleus (VLPO), also known as the intermediate nucleus of the preoptic area (IPA), is a small cluster of neurons situated in the anterior hypothalamus, sitting just above and to the side of the optic chiasm in the brain of humans and other animals.[1] The VLPO, together with the reticular activating system and the widely projecting orexin neuronal system that originates in the lateral hypothalamus, are the interconnected neural systems which control states of arousal, sleep, and transitions between these two states.[1] The VLPO is active during sleep, primarily during non-rapid eye movement sleep (NREM sleep), and releases inhibitory neurotransmitters, mainly GABA and galanin, which inhibit neurons of the reticular activating system that are involved in wakefulness and arousal.[1] The VLPO is in turn innervated by neurons from the aforementioned neural systems. The VLPO is activated by the sleep-inducing neurotransmitters serotonin and adenosine[2] and endosomnogen Prostaglandin D2.[3] The VLPO is inhibited during wakefulness by the arousal-inducing neurotransmitters norepinephrine and acetylcholine.[4] The role of the VLPO in sleep and wakefulness, and its association with sleep disorders – particularly insomnia and narcolepsy – is a growing area of neuroscience research.
Structure
Approximately 80% of neurons in the VLPO are GABAergic (neurons that produce GABA).[5] In vitro studies in rats have shown that two-thirds of the VLPO consists of homogeneous multipolar triangular shaped cells with low threshold spikes.[6] Approximately two thirds of these cells are active during sleep. All the triangular multipolar neurons are inhibited by norepinephrine and acetylcholine. However, in the past few years it has become clear that these triangular multipolar neurons exist in two sub-populations in the VLPO:
- Type 1 – inhibited by serotonin.
- Type 2 – excited by serotonin and adenosine.
Since serotonin and adenosine accumulate during wakefulness[2] it is likely that type 2 play a role in sleep induction, and type 1 play a role in sleep consolidation.
The remaining third of neurons in the VLPO are excited by norepinephrine. Their role is unclear.
Function
Sleep/wakefulness

In the mid-20th century in vivo studies – first in rats and then in cats – showed that lesions in the VLPO result in insomnia[7][8] suggesting the VLPO has a key role in sleep. VLPO innervates cell bodies and proximal dendrites of brain regions that are part of the arousal system; the tuberomammillary nucleus (TMN), the raphe nucleus, the locus coeruleus (LC), the pedunculopontine tegmental nuclei (PPT), the laterodorsal tegmental nucleus (LDT), the substantia nigra (SN), the ventral periaqueductal grey (vPAG) and the orexinergic neurons; all of these are monaminergic nuclei of diffuse modulatory systems (DMS).
The VLPO is activated by serotonin, adenosine and prostaglandin D2 which accumulate during wakefulness. A majority of VLPO neurons are GABAergic and galaninergic, primarily secreting the inhibitory neurotransmitters GABA and galanin respectively. The secretion of these inhibitory neurotransmitters suppresses the arousal system and results in sleep. The VLPO also receives afferent projections from the DMS nuclei of the arousal system. These afferent projections secrete the arousal neurotransmitters norepinephrine and acetylcholine which inhibit VLPO and result in wakefulness. VLPO firing is inversely proportionate to the activation of the arousal system (as VLPO firing increases, the arousal system firing decreases, and vice versa). A current theory suggests that VLPO and the arousal system exist in a simple A-B circuit, where A is inhibiting B and B is inhibiting A. This hypothesis, named the flip-flop switch model,[9] suggests that the counteracting inhibition between VLPO and the arousal system results in the brain switching between two states, the sleep state and the wake state, so that at any time only one is firing; VLPO or the arousal system. Orexin neurons in the posterior of the hypothalamus excite brain nuclei in the arousal system and help keep you awake. Orexin neurons also extend to the VLPO likely inhibiting its activity.[9] Thus orexin neurons are thought to play an important role in the stabilisation of the flip-flop switch regulating sleep and wakefulness.
Circadian rhythm
It has been suggested in recent years that sleep and circadian rhythms are closely linked in mammals. The “master clock” of circadian rhythms in mammals is the suprachiasmatic nucleus (SCN). The SCN has modest projections to the VLPO. These projections are thought to activate GABAergic neurons of the VLPO promoting the onset of sleep.[9] The VLPO in turn has projections to the SCN, suggesting the VLPO may play a role in the control of circadian rhythm in mammals.
Clinical significance
Insomnia
Elderly human patients with more galanin neurons in their intermediate nucleus (the human equivalent of the VLPO galanin neurons in rodents) have better, more continuous sleep. That is, a reduced number of these neurons is associated with more fragmented sleep (more awakenings throughout the night).[10]
Lesions in the VLPO in rats results in 50-60% decrease in NREM sleep time and prolonged insomnia.[11] It is hypothesised that disruption of the VLPO results in reduced and irregular inhibition of the arousal system by VLPO meaning patients with insomnia wake up several times during the night. More recent research suggests insomnia could be due to an imbalance of input to arousal system and VLPO neurons, including orexin neuron signalling. Orexin-receptor antagonists are currently being developed for the treatment of insomnia.[12]
Narcolepsy
Narcolepsy type I is due to a decrease in orexin. It is hypothesised in narcolepsy the reduced level of orexin means that the switch between the VLPO and the arousal system is destabilised [9] resulting in sudden switches from wakefulness to sleep; the characteristic symptom of narcoleptics.
Deep brain stimulation
In 2013 a novel method of deep brain stimulation, the magnetic field projector, which magnetically induces deep brain stimulation, was tested in rats and was shown to successfully modulate the firing activity of neurons in the VLPO, inducing sleep.[13] This new method of deep brain stimulation which avoids surgical operation and is much cheaper than current methods could lead to new therapies for insomnia and narcolepsy in humans.
Anaesthetics
Studies showed that two anaesthetics, isoflurane and halothane, increase the activity of the VLPO in mice.[14] This proved anaesthetics are capable of directly affecting sleep/wake networks, and indicates the potential of anaesthetics in the treatment of insomnia and narcolepsy. Propofol has been shown to increase activity in the VLPO [15] however the mechanism of action is uncertain.
References
- 1 2 3 Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 12: Sleep and Arousal". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 294–296. ISBN 9780071481274.
NEURAL SUBSTRATES OF SLEEP
Several neural systems mediate the switching between wakefulness and sleep and between the different stages of sleep 12–3. These systems include the ascending reticular activating system (ARAS), the ventrolateral pre-optic (VLPO) area, and the orexin/hypocretin system (Chapters 6 and 7). ... The VLPO area of the anterior hypothalamus consists mainly of inhibitory neurons that release γ-aminobutyric acid (GABA) and the neuropeptide galanin. The VLPO neurons are likely to have reciprocal interactions with the ARAS and orexin neurons. The VLPO neurons inhibit and are inhibited by the TMN histamine neurons and REM-off monoamine neurons. Orexin neurons are located in the lateral hypothalamus. They are organized in a widely projecting manner, much like the monoamines (Chapter 6), and innervate all of the components of the ARAS. They excite the REM-off monoaminergic neurons during wakefulness and the PT cholinergic neurons during REM sleep. They are inhibited by the VLPO neurons during NREM sleep. ... During NREM sleep, the VLPO area neurons start inhibiting the orexin neurons of the lateral hypothalamus. Consequently, the norepinephrine and serotonin REM-off cells, which are excited by orexin neurons during wakefulness, start to wane in activity, which gradually releases the cholinergic REM-on cells from their inhibitory effect. At the end of NREM sleep, the VLPO area neurons directly inhibit the REM-off cells, which completely disinhibits the REM-on cholinergic neurons and initiates REM sleep. Consistent with the inhibition of REMon cells by serotonergic and noradrenergic inputs, antidepressant drugs, which increase the availability of synaptic serotonin or norepinephrine (Chapter 14), reduce REM sleep. - 1 2 Gallopin T (2005). "The endogenous somnogen adenosine excites a subset of sleep-promoting neurons via A2A receptors in the ventrolateral preoptic nucleus". Neuroscience. 134 (4): 1377–1390. PMID 16039802. doi:10.1016/j.neuroscience.2005.05.045.
- ↑ Scammell T (1998). "Activation of ventrolateral preoptic neurons by the somnogen prostaglandin D2". PNAS. 95: 7754–7759. doi:10.1073/pnas.95.13.7754.
- ↑ Chou T (2002). "Afferents to the Ventrolateral Preoptic Nucleus". The Journal of Neuroscience. 22: 977–990.
- ↑ Sherin J (1998). "Innervation of Histaminergic Tuberomammillary Neurons by GABAergic and Galaninergic Neurons in the Ventrolateral Preoptic Nucleus of the Rat". The Journal of Neuroscience. 18 (12): 4705–4721.
- ↑ Gallopin T (2000). "Identification of sleep-promoting neurons in vitro". Nature. 404: 992–995. doi:10.1038/35010109.
- ↑ Nauta W (1946). "Hypothalamic regulation of sleep in rats". Journal of Neurophysiology. 9: 285–314.
- ↑ McGinty D (1968). "Sleep Suppression after Basal Forebrain Lesions in the Cat". Science. 160 (3833): 1253–1255. doi:10.1126/science.160.3833.1253.
- 1 2 3 4 Saper C (2005). "Hypothalamic regulation of sleep and circadian rhythms". Nature. 437 (7063): 1257–1263. PMID 16251950. doi:10.1038/nature04284.
- ↑ Lim A (2014). "Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer’s disease". Brain. 137: 2847–61.
- ↑ Liu J (2002). "Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep". The Journal of Neuroscience. 22: 4568–4576.
- ↑ Winrow C (2014). "Discovery and development of orexin receptor antagonists as therapeutics for insomnia". British Journal of Pharmacology. 171: 283–293. doi:10.1111/bph.12261.
- ↑ Jie F (2013). "A magnetic field projector for deep brain modulation". Neural Engineering: 1214–1217. doi:10.1109/NER.2013.6696158.
- ↑ Moore J (2012). "Direct Activation of Sleep-Promoting VLPO Neurons by Volatile Anesthetics Contributes to Anesthetic Hypnosis". Current Biology. 22: 2008–2016. doi:10.1016/j.cub.2012.08.042.
- ↑ Liu Y (2013). "Propofol Facilitates Glutamatergic Transmission to Neurons of the Ventrolateral Preoptic Nucleus". Anesthesiology. 111: 1271–1278. doi:10.1097/ALN.0b013e3181bf1d79.
External links
Gallopin T, Luppi PH, Cauli B, Urade Y, Rossier J, Hayaishi O, Lambolez B, Fort P (2005). "The endogenous somnogen adenosine excites a subset of sleep-promoting neurons via A2A receptors in the ventrolateral preoptic nucleus". Neuroscience. 134 (4): 1377–90. PMID 16039802. doi:10.1016/j.neuroscience.2005.05.045.