Ubiquitin
| Ubiquitin family | |||||||||
|---|---|---|---|---|---|---|---|---|---|
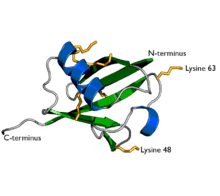 A diagram of ubiquitin. The seven lysine sidechains are shown in yellow/orange. | |||||||||
| Identifiers | |||||||||
| Symbol | ubiquitin | ||||||||
| Pfam | PF00240 | ||||||||
| InterPro | IPR000626 | ||||||||
| PROSITE | PDOC00271 | ||||||||
| SCOP | 1aar | ||||||||
| SUPERFAMILY | 1aar | ||||||||
| |||||||||
Ubiquitin is a small (8.5 kDa) regulatory protein found in most tissues of eukaryotic organisms i.e. it occurs ubiquitously. It was discovered in 1975[1] in Israel by Gideon Goldstein and further characterized throughout the 1970s and 1980s.[2] Four genes in the human genome code for ubiquitin: UBB, UBC, UBA52 and RPS27A.[3]
The addition of ubiquitin to a substrate protein is called ubiquitination or less frequently ubiquitylation. Ubiquitination affects proteins in many ways: it can mark them for degradation via the proteasome, alter their cellular location, affect their activity, and promote or prevent protein interactions.[4][5][6] Ubiquitination involves three main steps: activation, conjugation, and ligation, performed by ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s), and ubiquitin ligases (E3s), respectively. The result of this sequential cascade is to bind ubiquitin to lysine residues on the protein substrate via an isopeptide bond, cysteine residues through a thioester bond, serine and threonine residues through an ester bond, or the amino group of the protein's N-terminus via a peptide bond.[7][8][9]
The protein modifications can be either a single ubiquitin protein (monoubiquitination) or a chain of ubiquitin (polyubiquitination). Secondary ubiquitin molecules are always linked to one of the seven lysine residues or the N-terminal methionine of the previous ubiquitin molecule. These 'linking' residues are represented by a "K" or "M" (the one-letter amino acid notation of lysine and methionine, respectively) and a number, referring to its position in the ubiquitin molecule as in K48, K29 or M1. The first ubiquitin molecule is covalently bound through its C-terminal carboxylate group to a particular lysine, cysteine, serine, threonine or N-terminus of the target protein. Poly-ubiquitination occurs when the C-terminus of another ubiquitin, is then linked to one of the seven lysine residues or the first methionine on the previously added ubiquitin molecule, creating a chain. This process repeats several times, leading to the addition of several ubiquitins. Only poly-ubiquitination on defined lysines, mostly on K48 and K29, is related to degradation by the proteasome (referred to as the "molecular kiss of death"), while other polyubiquitinations (e.g. on K63, K11, K6 and M1) and monoubiquitinations may regulate processes such as endocytic trafficking, inflammation, translation and DNA repair.[10]
The discovery that ubiquitin chains target proteins to the proteasome, which degrades and recycles proteins, was honored with the Nobel Prize in chemistry in 2004.[8][11][12]
Identification

Ubiquitin (originally, ubiquitous immunopoietic polypeptide) was first identified in 1975[1] as an 8.5 kDa protein of unknown function expressed in all eukaryotic cells. The basic functions of ubiquitin and the components of the ubiquitination pathway were elucidated in the early 1980s at the Technion by Aaron Ciechanover, Avram Hershko, and Irwin Rose for which the Nobel Prize in Chemistry was awarded in 2004.[11]
The ubiquitination system was initially characterised as an ATP-dependent proteolytic system present in cellular extracts. A heat-stable polypeptide present in these extracts, ATP-dependent proteolysis factor 1 (APF-1), was found to become covalently attached to the model protein substrate lysozyme in an ATP- and Mg2+-dependent process.[13] Multiple APF-1 molecules were linked to a single substrate molecule by an isopeptide linkage, and conjugates were found to be rapidly degraded with the release of free APF-1. Soon after APF-1-protein conjugation was characterised, APF-1 was identified as ubiquitin. The carboxyl group of the C-terminal glycine residue of ubiquitin (Gly76) was identified as the moiety conjugated to substrate lysine residues.
The protein
| Number of residues | 76 |
| Molecular mass | 8564.8448 Da |
| Isoelectric point (pI) | 6.79 |
| Gene names | RPS27A (UBA80, UBCEP1), UBA52 (UBCEP2), UBB, UBC |
| Sequence in amino acid abbreviations | MQIFVKTLTGKTITLEVEPSDTIENVKAKIQDKEGIPPD
QQRLIFAGKQLEDGRTLSDYNIQKESTLHLVLRLRGG |
Ubiquitin is a small protein that exists in all eukaryotic cells. It performs its myriad functions through conjugation to a large range of target proteins. A variety of different modifications can occur. The ubiquitin protein itself consists of 76 amino acids and has a molecular mass of about 8.5 kDa. Key features include its C-terminal tail and the 7 lysine residues. It is highly conserved among eukaryotic species: Human and yeast ubiquitin share 96% sequence identity.
Genes
Ubiquitin is encoded in mammals by 4 different genes. UBA52 and RPS27A genes code for a single copy of ubiquitin fused to the ribosomal proteins L40 and S27a, respectively. The UBB and UBC genes code for polyubiquitin precursor proteins.[3]
Origins
No ubiquitin and ubiquitination machinery are known to exist in prokaryotes. However, ubiquitin is believed to have descended from prokaryotic proteins similar to ThiS[14] or MoaD.[15] These prokaryotic proteins, despite having little sequence identity (ThiS has 14% identity to ubiquitin), share the same protein fold. These proteins also share sulfur chemistry with ubiquitin. MoaD, which is involved in molybdenum cofactor biosynthesis, interacts with MoeB, which acts like an E1 ubiquitin-activating enzyme for MoaD, strengthening the link between these prokaryotic proteins and the ubiquitin system. A similar system exists for ThiS, with its E1-like enzyme ThiF. It is also believed that the Saccharomyces cerevisiae protein Urm-1, a ubiquitin-related modifier, is a "molecular fossil" that connects the evolutionary relation with the prokaryotic ubiquitin-like molecules and ubiquitin.[16]
Ubiquitination
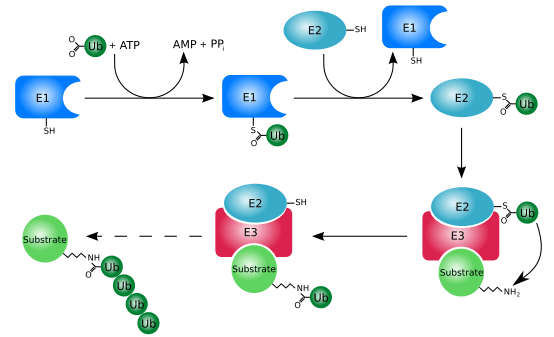
Ubiquitination (or ubiquitylation) is an enzymatic post-translational modification in which a ubiquitin protein is attached to a substrate protein. This process most commonly binds the last amino acid of ubiquitin (glycine 76) to a lysine residue on the substrate. An isopeptide bond is formed between the carboxyl group (COO−) of the ubiquitin's glycine and the epsilon-amino group (ε-NH+
3) of the substrate's lysine.[17] Trypsin cleavage of a ubiquitin-conjugated substrate leaves a di-glycine "remnant" that is used to identify the site of ubiquitination.[18][19] Ubiquitin can also be bound to other sites in a protein which are electron-rich nucleophiles, termed "non-canonical ubiquitination".[9] This was first observed with the amine group of a protein's N-terminus being used for ubiquitination, rather than a lysine residue, in the protein MyoD[20] and has been observed since in 22 other proteins in multiple species,[21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][22][38][39] including ubiquitin itself.[40][41] There is also increasing evidence for nonlysine residues as ubiquitination targets using non-amine groups, such as the sulfhydryl group on cysteine,[36][37][42][43][44][45][46][47][48][49] and the hydroxyl group on threonine and serine.[36][37][42][48][49][50][51][52][53] The end result of this process is the addition of one ubiquitin molecule (monoubiquitination) or a chain of ubiquitin molecules (polyubiquitination) to the substrate protein.[54]
Ubiquitination requires three types of enzyme: ubiquitin-activating enzymes, ubiquitin-conjugating enzymes, and ubiquitin ligases, known as E1s, E2s, and E3s, respectively. The process consists of three main steps:
- Activation: Ubiquitin is activated in a two-step reaction by an E1 ubiquitin-activating enzyme, which is dependent on ATP. The initial step involves production of a ubiquitin-adenylate intermediate. The E1 binds both ATP and ubiquitin and catalyses the acyl-adenylation of the C-terminus of the ubiquitin molecule. The second step transfers ubiquitin to an active site cysteine residue, with release of AMP. This step results in a thioester linkage between the C-terminal carboxyl group of ubiquitin and the E1 cysteine sulfhydryl group.[17][55] The human genome contains two genes that produce enzymes capable of activating ubiquitin: UBA1 and UBA6.[56]
- Conjugation: E2 ubiquitin-conjugating enzymes catalyse the transfer of ubiquitin from E1 to the active site cysteine of the E2 via a trans(thio)esterification reaction. In order to perform this reaction, the E2 binds to both activated ubiquitin and the E1 enzyme. Humans possess 35 different E2 enzymes, whereas other eukaryotic organisms have between 16 and 35. They are characterised by their highly conserved structure, known as the ubiquitin-conjugating catalytic (UBC) fold.[57]
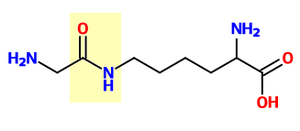 Glycine and lysine linked by an isopeptide bond. The isopeptide bond is highlighted yellow.
Glycine and lysine linked by an isopeptide bond. The isopeptide bond is highlighted yellow. - Ligation: E3 ubiquitin ligases catalyse the final step of the ubiquitination cascade. Most commonly, they create an isopeptide bond between a lysine of the target protein and the C-terminal glycine of ubiquitin. In general, this step requires the activity of one of the hundreds of E3s. E3 enzymes function as the substrate recognition modules of the system and are capable of interaction with both E2 and substrate. Some E3 enzymes also activate the E2 enzymes. E3 enzymes possess one of two domains: the homologous to the E6-AP carboxyl terminus (HECT) domain and the really interesting new gene (RING) domain (or the closely related U-box domain). HECT domain E3s transiently bind ubiquitin in this process (an obligate thioester intermediate is formed with the active-site cysteine of the E3), whereas RING domain E3s catalyse the direct transfer from the E2 enzyme to the substrate.[58] The anaphase-promoting complex (APC) and the SCF complex (for Skp1-Cullin-F-box protein complex) are two examples of multi-subunit E3s involved in recognition and ubiquitination of specific target proteins for degradation by the proteasome.[59]
In the ubiquitination cascade, E1 can bind with many E2s, which can bind with hundreds of E3s in a hierarchical way. Having levels within the cascade allows tight regulation of the ubiquitination machinery.[7] Other ubiquitin-like proteins (UBLs) are also modified via the E1–E2–E3 cascade, although variations in these systems do exist.[60]
E4 enzymes, or ubiquitin-chain elongation factors, are capable of adding pre-formed polyubiquitin chains to substrate proteins.[61] For example, multiple monoubiquitylation of the tumor suppressor p53 by Mdm2[62] can be followed by addition of a polyubiquitin chain using p300 and CBP.[63][64]
Types
Ubiquitination affects cellular process by regulating the degradation of proteins (via the proteasome and lysosome), coordinating the cellular localization of proteins, activating and inactivating proteins, and modulating protein-protein interactions.[4][5][6] These effects are mediated by different types of substrate ubiquitination, for example the addition of a single ubiquitin molecule (monoubiquitination) or different types of ubiquitin chains (polyubiquitination).[65]
Monoubiquitination
Monoubiquitination is the addition of one ubiquitin molecule to one substrate protein residue. Multi-monoubiquitination is the addition of one ubiquitin molecule to multiple substrate residues. The monoubiquitination of a protein can have different effects to the polyubiquitination of the same protein. The addition of a single ubiquitin molecule is thought to be required prior to the formation of polyubiquitin chains.[65] Monoubiquitination affects cellular processes such as membrane trafficking, endocytosis and viral budding.[10][66]
Polyubiquitin chains
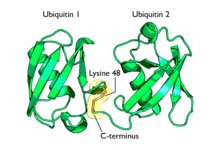
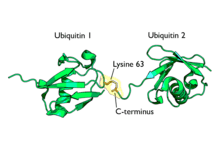
Polyubiquitination is the formation of a ubiquitin chain on a single lysine residue on the substrate protein. Following addition of a single ubiquitin moiety to a protein substrate, further ubiquitin molecules can be added to the first, yielding a polyubiquitin chain.[65] These chains are made by linking the glycine residue of a ubiquitin molecule to a lysine of ubiquitin bound to a substrate. Ubiquitin has seven lysine residues and an N-terminus that serves as points of ubiquitination; they are K6, K11, K27, K29, K33, K48, K63 and M1, respectively.[8] Lysine 48-linked chains were the first identified and are the best-characterised type of ubiquitin chain. K63 chains have also been well-characterised, whereas the function of other lysine chains, mixed chains, branched chains, M1-linked linear chains, and heterologous chains (mixtures of ubiquitin and other ubiquitin-like proteins) remains more unclear.[19][41][65][66][67]
Lysine 48-linked polyubiquitin chains target proteins for destruction, by a process known as proteolysis. At least four ubiquitin molecules must be attached to a lysine residue on the condemned protein in order for it to be recognised by the 26S proteasome.[68] This is a barrel-shape structure comprising a central proteolytic core made of four ring structures, flanked by two cylinders that selectively allow entry of ubiquitinated proteins. Once inside, the proteins are rapidly degraded into small peptides (usually 3–25 amino acid residues in length). Ubiquitin molecules are cleaved off the protein immediately prior to destruction and are recycled for further use.[69] Although the majority of protein substrates are ubiquitinated, there are examples of non-ubiquitinated proteins targeted to the proteasome.[70] The polyubiquitin chains are recognised by a subunit of the proteasome: S5a/Rpn10. This is achieved by a ubiquitin interacting motif (UIM) found in a hydrophobic patch in the C-terminal region of the S5a/Rpn10 unit.[4]
Lysine 63-linked chains are not associated with proteasomal degradation of the substrate protein. Instead, they allow the coordination of other processes such as endocytic trafficking, inflammation, translation, and DNA repair.[10] In cells, lysine 63-linked chains are bound by the ESCRT-0 complex, which prevents their binding to the proteasome. This complex contains two proteins, Hrs and STAM1, that contain a UIM, which allows it to bind to lysine 63-linked chains.[71][72]
Less is understood about atypical (non-lysine 48-linked) ubiquitin chains but research is starting to suggest roles for these chains.[66] There is evidence to suggest that atypical chains linked by lysine 6, 11, 27, 29 and methionine 1 can induce proteasomal degradation.[70][73]
Branched ubiquitin chains containing multiple linkage types can be formed.[74] The function of these chains is unknown.[8]
Structure
Differently linked chains have specific effects on the protein to which they are attached, caused by differences in the conformations of the protein chains. K29-, K33-,[75] K63- and M1-linked chains have a fairly linear conformation; they are known as open-conformation chains. K6-, K11-, and K48-linked chains form closed conformations. The ubiquitin molecules in open-conformation chains do not interact with each other, except for the covalent isopeptide bonds linking them together. In contrast, the closed conformation chains have interfaces with interacting residues. Altering the chain conformations exposes and conceals different parts of the ubiquitin protein, and the different linkages are recognized by proteins that are specific for the unique topologies that are intrinsic to the linkage. Proteins can specifically bind to ubiquitin via ubiquitin-binding domains (UBDs). The distances between individual ubiquitin units in chains differ between lysine 63- and 48-linked chains. The UBDs exploit this by having small spacers between ubiquitin-interacting motifs that bind lysine 48-linked chains (compact ubiquitin chains) and larger spacers for lysine 63-linked chains. The machinery involved in recognising polyubiquitin chains can also differentiate between K63-linked chains and M1-linked chains, demonstrated by the fact that the latter can induce proteasomal degradation of the substrate.[8][10][73]
Function
The ubiquitination system functions in a wide variety of cellular processes, including:[76]
- Antigen processing
- Apoptosis
- Biogenesis of organelles
- Cell cycle and division
- DNA transcription and repair
- Differentiation and development
- Immune response and inflammation
- Neural and muscular degeneration
- Morphogenesis of neural networks
- Modulation of cell surface receptors, ion channels and the secretory pathway
- Response to stress and extracellular modulators
- Ribosome biogenesis
- Viral infection
Membrane proteins
Multi-monoubiquitination can mark transmembrane proteins (for example, receptors) for removal from membranes (internalisation) and fulfil several signalling roles within the cell. When cell-surface transmembrane molecules are tagged with ubiquitin, the subcellular localization of the protein is altered, often targeting the protein for destruction in lysosomes. This serves as a negative feedback mechanism because often the stimulation of receptors by ligands increases their rate of ubiquitination and internalisation. Like monoubiquitination, lysine 63-linked polyubiquitin chains also has a role in the trafficking some membrane proteins.[10][65][68]
Genomic maintenance
Proliferating cell nuclear antigen (PCNA) is a protein involved in DNA synthesis. Under normal physiological conditions PCNA is sumoylated (a similar post-translational modification to ubiquitination). When DNA is damaged by ultra-violet radiation or chemicals, the SUMO molecule that is attached to a lysine residue is replaced by ubiquitin. Monoubiquitinated PCNA recruits polymerases that can carry out DNA synthesis with damaged DNA; but this is very error-prone, possibly resulting in the synthesis of mutated DNA. Lysine 63-linked polyubiquitination of PCNA allows it to perform a less error-prone mutation bypass known by the template switching pathway.[6][77][78]
Ubiquitination of histone H2AX is involved in DNA damage recognition of DNA double-strand breaks. Lysine 63-linked polyubiquitin chains are formed on H2AX histone by the E2/E3 ligase pair, Ubc13-Mms2/RNF168.[79][80] This K63 chain appears to recruit RAP80, which contains a UIM, and RAP80 then helps localize BRCA1. This pathway will eventually recruit the necessary proteins for homologous recombination repair.[81]
Transcriptional regulation
Histones can be ubiquitinated and this is usually in the form of monoubiquitination (although polyubiquitinated forms do occur). Histone ubiquitination alters chromatin structure and allows the access of enzymes involved in transcription. Ubiquitin on histones also acts as a binding site for proteins that either activate or inhibit transcription and also can induce further post-translational modifications of the protein. These effects can all modulate the transcription of genes.[82][83]
Deubiquitination
Deubiquitinating enzymes (DUBs) oppose the role of ubiquination by removing ubiquitin from substrate proteins. They are cysteine proteases that cleave the amide bond between the two proteins. They are highly specific, as are the E3 ligases that attach the ubiquitin, with only a few substrates per enzyme. They can cleave both isopeptide (between ubiquitin and lysine) and peptide bonds (between ubiquitin and the N-terminus). In addition to removing ubiquitin from substrate proteins, DUBs have many other roles within the cell. Ubiquitin is either expressed as multiple copies joined in a chain (polyubiquitin) or attached to ribosomal subunits. DUBs cleave these proteins to produce active ubiquitin. They also recycle ubiquitin that has been bound to small nucleophilic molecules during the ubiquitination process. Monoubiquitin is formed by DUBs that cleave ubiquitin from free polyubiquitin chains that have been previously removed from proteins.[84][85]
Ubiquitin-binding domains
| Domain | Number of Proteins
in Proteome |
Length
(amino acids) |
Ubiquitin Binding
Affinity |
|---|---|---|---|
| CUE | S. cerevisiae 7
H. sapiens 21 |
42–43 | ~2–160 μM |
| GATII | S. cerevisiae 2
H. sapiens 14 |
135 | ~180 μM |
| GLUE | S. cerevisiae ?
H. sapiens ? |
~135 | ~460 μM |
| NZF | S. cerevisiae 1
H. sapiens 25 |
~35 | ~100–400 μM |
| PAZ | S. cerevisiae 5
H. sapiens 16 |
~58 | Not known |
| UBA | S. cerevisiae 10
H. sapiens 98 |
45–55 | ~0.03-500 μM |
| UEV | S. cerevisiae 2
H. sapiens ? |
~145 | ~100–500 μM |
| UIM | S. cerevisiae 8
H. sapiens 71 |
~20 | ~100–400 μM |
| VHS | S. cerevisiae 4
H. sapiens 28 |
150 | Not known |
Ubiquitin-binding domains (UBDs) are modular protein domains that non-covalently bind to ubiquitin, these motifs control various cellular events. Detailed molecular structures are known for a number of UBDs, binding specificity determines their mechanism of action and regulation, and how it regulates cellular proteins and processes.[86][87]
Disease associations
Pathogenesis
The ubiquitin pathway has been implicated in the pathogenesis of several diseases and genetic disorders:
- Neurodegenerative disorders: Transcript variants encoding different isoforms of ubiquilin-1 are found in lesions associated with Alzheimer's and Parkinson's disease.[88] Higher levels of ubiquilin in the brain have been shown to decrease malformation of amyloid precursor protein (APP), which plays a key role in triggering Alzheimer's disease.[89] Conversely, lower levels of ubiquilin-1 in the brain have been associated with increased malformation of APP.[89] A frameshift mutation in ubiquitin B can result in a truncated peptide missing the C-terminal glycine. This abnormal peptide, known as UBB+1, has been shown to accumulate selectively in Alzheimer's disease and other tauopathies.
- Angelman syndrome is caused by a disruption of UBE3A, which encodes a ubiquitin ligase (E3) enzyme termed E6-AP.
- Von Hippel-Lindau syndrome involves disruption of a ubiquitin E3 ligase termed the VHL tumor suppressor, or VHL gene.
- Fanconi anemia: Eight of the thirteen identified genes whose disruption can cause this disease encode proteins that form a large ubiquitin ligase (E3) complex.
- 3-M syndrome is an autosomal-recessive growth retardation disorder associated with mutations of the Cullin7 E3 ubiquitin ligase.[90]
Diagnostic use
Immunohistochemistry using antibodies to ubiquitin can identify abnormal accumulations of this protein inside cells, indicating a disease process. These protein accumulations are referred to as inclusion bodies (which is a general term for any microscopically visible collection of abnormal material in a cell). Examples include:
- Neurofibrillary tangles in Alzheimer's disease
- Lewy body in Parkinson's disease
- Pick bodies in Pick's disease
- Inclusions in motor neuron disease and Huntington's Disease
- Mallory bodies in alcoholic liver disease
- Rosenthal fibers in astrocytes
Link to cancer
Post-translational modification of proteins is a generally used mechanism in eukaryotic cell signaling.[91] Ubiquitination, or ubiquitin conjugation to proteins, is a crucial process for cell cycle progression and cell proliferation and development. Although ubiquitination usually serves as a signal for protein degradation through the 26S proteasome, it could also serve for other fundamental cellular processes,[91] e.g. in endocytosis,[92] enzymatic activation[93] and DNA repair.[94] Moreover, since ubiquitination functions to tightly regulate the cellular level of cyclins, its misregulation is highly expected to have severe impacts. First evidence of the importance of the ubiquitin/proteasome pathway in oncogenic processes was observed due to the high antitumor activity of proteasome inhibitors.[95][96][97] Various studies have shown that defects or alterations in ubiquitination processes are commonly associated with or presence in human carcinoma.[98][99][100][101][102][103][104][105] Malignancies could be developed through loss of function mutation directly at the tumor suppressor gene, increased activity of ubiquitination, and/or indirect attenuation of ubiquitination due to mutation in related proteins.[106]
Direct loss of function mutation of E3 ubiquitin ligase
Renal cell carcinoma
The VHL (Von Hippel–Lindau) gene encodes a component of an E3 Ubiquitin Ligase. VHL complex targets member of the hypoxia-inducible transcription factor family (HIF) for degradation by interacting with the oxygen-dependent destruction domain under normoxic condition. HIF activates downstream targets such as the vascular endothelial growth factor (VEGF), promoting angiogenesis. Mutations in VHL prevent degradation of HIF and thus lead to the formation of hypervascular lesions and renal tumors.[98][106]
Breast cancer
The BRCA1 gene is another tumor suppressor gene in human which encodes the BRCA1 protein that is involved in response to DNA damage. The protein contains a RING motif with E3 Ubiquitin Ligase activity. BRCA1 could form dimer with other molecules, such as BARD1 and BAP1, for its ubiquitination activity. Mutations that affect the ligase function are often found and associated with various cancers.[102][106]
Cyclin E
As processes in cell cycle progression is the most fundamental processes for cellular growth and differentiation, and are the most common to be altered in human carcinomas, it is expected for cell cycle-regulatory proteins to be under tight regulation. The level of cyclins, as the name suggests, are high only at certain time point during cell cycle. This is achieved by continuous controlled of cyclins/CDKs level through ubiquitination and degradation. When cyclin E is partnered with CDK2 and get phosphorylated, an SCF-associated F-box protein Fbw7 recognizes the complex and thus target it for degradation. Mutations in Fbw7 have been found in more than 30% of human tumors, characterizing is as a tumor suppressor protein.[105]
Increased ubiquitination activity
Cervical cancer
Oncogenic types of the human papillomavirus (HPV) are known to hijack cellular ubiquitin-proteasome pathway for viral infection and replication. The E6 proteins of HPV will bind to the N-terminus of the cellular E6-AP E3 ubiquitin ligase, redirecting the complex to bind p53, a well-known tumor suppressor gene that inactivation is found in many types of cancer.[100] Thus, p53 undergoes ubiquitination and proteasome-mediated degradation. Meanwhile, E7, another one of the early-expressed HPV genes, will bind to Rb, also a tumor suppressor gene, mediating its degradation.[106] The loss of p53 and Rb in cells allows limitless cell proliferation to occur.
p53 regulation
Gene amplification often occur in various tumor cases, including of MDM2, a gene encodes for a RING E3 Ubiquitin ligase responsible for downregulation of p53 activity. MDM2 targets p53 for ubiquitination and proteasomal degradation thus keeping its level appropriate for normal cell condition. Overexpression of MDM2 causes loss of p53 activity and therefore allowing cells to have a limitless replicative potential.[101][106]
p27
Another gene that is a target of gene amplification is SKP2. SKP2 is an F-box protein that roles in substrate recognition for ubiquitination and degradation. SKP2 targets p27Kip-1, an inhibitor of cyclin-dependent kinases (CDKs). CDKs2/4 partner with the cyclinsE/D, respectively, family of cell cycle regulator to control cell cycle progression through the G1 phase. Low level of p27Kip-1 protein is often found in various cancers and is due to overactivation of ubiquitin-mediated proteolysis through overexpression of SKP2.[103][106]
Efp
Efp, or estrogen-inducible RING-finger protein, is an E3 ubiquitin ligase that overexpression has been shown to be the major cause of estrogen-independent breast cancer.[97][107] Efp's substrate is 14-3-3 protein which negatively regulates cell cycle.
Evasion of Ubiquitination
Colorectal cancer
The gene associated with colorectal cancer is the adenomatous polyposis coli (APC), which is a classic tumor suppressor gene. APC gene product targets beta-catenin for degradation via ubiquitination at the N-terminus, thus regulating its cellular level. Most colorectal cancer cases are found with mutations in the APC gene. However, in cases where APC gene is not mutated, mutations are found in the N-terminus of beta-catenin which renders it ubiquitination-free and thus increased activity.[99][106]
Glioblastoma
As the most aggressive cancer originated in the brain, mutations found in patients with glioblastoma are related to the deletion of a part of the extracellular domain of the epidermal growth factor receptor (EGFR). This deletion causes CBL E3 ligase unable to bind the receptor for its recycling and degradation via a ubiquitin-lysosomal pathway. Thus, EGFR is constitutively active in the cell membrane and activates its downstream effectors that are involved in cell proliferation and migration.[104]
Phosphorylation-dependent ubiquitination
The interplay between ubiquitination and phosphorylation has been an ongoing research interest since phosphorylation often serves as a marker where ubiquitination leads to degradation.[91] Moreover, ubiquitination can also act to turn on/off the kinase activity of a protein.[108] The critical role of phosphorylation is largely underscored in the activation and removal of autoinhibition in Cbl protein.[109] Cbl is an E3 ubiquitin ligase with a RING finger domain that interacts with its tyrosine kinase binding (TKB) domain, preventing interaction of the RING domain with an E2 ubiquitin-conjugating enzyme. This intramolecular interaction is an autoinhibition regulation that prevents its role as a negative regulator of various growth factors and tyrosine kinase signaling and T-cell activation.[109] Phosphorylation of Y363 relieves the autoinhibition and enhances binding to E2.[109] Mutations that renders the Cbl protein dysfunctional due to the loss of its ligase/tumor suppressor function and maintenance of its positive signaling/oncogenic function have been shown to cause development of cancer.[110][111]
As a drug target
Screening for ubiquitin ligase substrates
Identification of E3 ligase substrates is critical to understand its implication in human diseases since deregulation of E3-substrate interactions are often served as major cause in many. To overcome the limitation of mechanism used to identify the substrates of the E3 Ubiquitin Ligase, a system called the 'Global Protein Stability (GPS) Profiling' was developed in 2008.[112] This high-throughput system made use of reporter proteins fused with thousands of potential substrates independently. By inhibition of the ligase activity (through the making of Cul1 dominant negative thus renders ubiquitination not to occur), increased reporter activity shows that the identified substrates are being accumulated. This approach added a large number of new substrates to the list of E3 ligase substrates.
Possible therapeutic appllications
Blocking of specific substrate recognition by the E3 ligases, e.g. Bortezomib.[107]
Challenge
Finding a specific molecule that selectively inhibits the activity of a certain E3 ligase and/or the protein-protein interactions implicated in the disease remains as one of the important and expanding research area. Moreover, as ubiquitination is a multi-step process with various players and intermediate forms, consideration of the much complex interactions between components needs to be taken heavily into account while designing the small molecule inhibitors.[97]
Ubiquitin-like modifiers
Although ubiquitin is the most-understood post-translation modifier, there is a growing family of ubiquitin-like proteins (UBLs) that modify cellular targets in a pathway that is parallel to, but distinct from, that of ubiquitin. Known UBLs include: small ubiquitin-like modifier (SUMO), ubiquitin cross-reactive protein (UCRP, also known as interferon-stimulated gene-15 ISG15), ubiquitin-related modifier-1 (URM1), neuronal-precursor-cell-expressed developmentally downregulated protein-8 (NEDD8, also called Rub1 in S. cerevisiae), human leukocyte antigen F-associated (FAT10), autophagy-8 (ATG8) and -12 (ATG12), Few ubiquitin-like protein (FUB1), MUB (membrane-anchored UBL),[113] ubiquitin fold-modifier-1 (UFM1) and ubiquitin-like protein-5 (UBL5, which is but known as homologous to ubiquitin-1 [Hub1] in S. pombe).[114][115] Whilst these proteins share only modest primary sequence identity with ubiquitin, they are closely related three-dimensionally. For example, SUMO shares only 18% sequence identity, but they contain the same structural fold. This fold is called "ubiquitin fold" or sometimes called ubiquitin fold. FAT10 and UCRP contain two. This compact globular beta-grasp fold is found in ubiquitin, UBLs, and proteins that comprise a ubiquitin-like domain, e.g. the S. cerevisiae spindle pole body duplication protein, Dsk2, and NER protein, Rad23, both contain N-terminal ubiquitin domains.
These related molecules have novel functions and influence diverse biological processes. There is also cross-regulation between the various conjugation pathways, since some proteins can become modified by more than one UBL, and sometimes even at the same lysine residue. For instance, SUMO modification often acts antagonistically to that of ubiquitination and serves to stabilize protein substrates. Proteins conjugated to UBLs are typically not targeted for degradation by the proteasome but rather function in diverse regulatory activities. Attachment of UBLs might, alter substrate conformation, affect the affinity for ligands or other interacting molecules, alter substrate localization, and influence protein stability.
UBLs are structurally similar to ubiquitin and are processed, activated, conjugated, and released from conjugates by enzymatic steps that are similar to the corresponding mechanisms for ubiquitin. UBLs are also translated with C-terminal extensions that are processed to expose the invariant C-terminal LRGG. These modifiers have their own specific E1 (activating), E2 (conjugating) and E3 (ligating) enzymes that conjugate the UBLs to intracellular targets. These conjugates can be reversed by UBL-specific isopeptidases that have similar mechanisms to that of the deubiquitinating enzymes.[76]
Within some species, the recognition and destruction of sperm mitochondria through a mechanism involving ubiquitin is responsible for sperm mitochondria's disposal after fertilization occurs.[116]
Prokaryotic ubiquitin-like protein (Pup) and ubiquitin bacterial (UBact)
Prokaryotic ubiquitin-like protein (Pup) is a functional analog of ubiquitin which has been found in the gram-positive bacterial phylum Actinobacteria. It serves the same function (targeting proteins for degradations), although the enzymology of ubiquitination and pupylation is different. In contrast to the three-step reaction of ubiquitination, pupylation requires two steps, therefore only two enzymes are involved in pupylation.
In 2017, homologs of Pup were reported in five phyla of gram-negative bacteria, in seven candidate bacterial phyla and in one archaeon[117] The sequences of the Pup homologs are very different from the sequences of Pup in gram-positive bacteria and were termed UBact (for Ubiquitin bacterial), although the distinction has yet not been proven to be phylogenetically supported by a separate evolutionary origin and is without experimental evidence[117].
The finding of the Pup/UBact-proteasome system in both gram-positive and gram-negative bacteria suggests that either the Pup/UBact-proteasome system evolved in bacteria prior to the split into gram positive and negative clades over 3000 million years ago[14] or, that these systems were acquired by different bacterial lineages through horizontal gene transfer(s) from a third, yet unknown, organism. In support of the second possibility, two UBact loci were found in the genome of an uncultured anaerobic methanotrophic Archaeon (ANME-1;locus CBH38808.1 and locus CBH39258.1).
Human proteins containing ubiquitin domain
ANUBL1; BAG1; BAT3/BAG6; C1orf131; DDI1; DDI2; FAU; HERPUD1; HERPUD2; HOPS; IKBKB; ISG15; LOC391257; MIDN; NEDD8; OASL; PARK2; RAD23A; RAD23B; RPS27A; SACS; 8U SF3A1; SUMO1; SUMO2; SUMO3; SUMO4; TMUB1; TMUB2; UBA52; UBB; UBC; UBD; UBFD1; UBL4; UBL4A; UBL4B; UBL7; UBLCP1; UBQLN1; UBQLN2; UBQLN3; UBQLN4; UBQLNL; UBTD1; UBTD2; UHRF1; UHRF2;
Related proteins
Prediction of ubiquitination
Currently available prediction programs are:
- UbiPred is a SVM-based prediction server using 31 physicochemical properties for predicting ubiquitination sites.[118]
- UbPred is a random forest-based predictor of potential ubiquitination sites in proteins. It was trained on a combined set of 266 non-redundant experimentally verified ubiquitination sites available from our experiments and from two large-scale proteomics studies.[119]
- CKSAAP_UbSite is SVM-based prediction that employs the composition of k-spaced amino acid pairs surrounding a query site (i.e. any lysine in a query sequence) as input, uses the same dataset as UbPred.[120]
See also
References
- 1 2 Goldstein G, Scheid M, Hammerling U, Schlesinger DH, Niall HD, Boyse EA (January 1975). "Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells". Proceedings of the National Academy of Sciences of the United States of America. 72 (1): 11–5. PMC 432229
 . PMID 1078892. doi:10.1073/pnas.72.1.11.
. PMID 1078892. doi:10.1073/pnas.72.1.11. - ↑ Wilkinson KD (October 2005). "The discovery of ubiquitin-dependent proteolysis". Proceedings of the National Academy of Sciences of the United States of America. 102 (43): 15280–2. PMC 1266097
 . PMID 16230621. doi:10.1073/pnas.0504842102.
. PMID 16230621. doi:10.1073/pnas.0504842102. - 1 2 Kimura Y, Tanaka K (June 2010). "Regulatory mechanisms involved in the control of ubiquitin homeostasis". Journal of Biochemistry. 147 (6): 793–8. PMID 20418328. doi:10.1093/jb/mvq044.
- 1 2 3 Glickman MH, Ciechanover A (April 2002). "The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction". Physiological Reviews. 82 (2): 373–428. PMID 11917093. doi:10.1152/physrev.00027.2001.
- 1 2 Mukhopadhyay D, Riezman H (January 2007). "Proteasome-independent functions of ubiquitin in endocytosis and signaling". Science. 315 (5809): 201–5. PMID 17218518. doi:10.1126/science.1127085.
- 1 2 3 Schnell JD, Hicke L (September 2003). "Non-traditional functions of ubiquitin and ubiquitin-binding proteins". The Journal of Biological Chemistry. 278 (38): 35857–60. PMID 12860974. doi:10.1074/jbc.R300018200.
- 1 2 Pickart CM, Eddins MJ (November 2004). "Ubiquitin: structures, functions, mechanisms". Biochimica et Biophysica Acta. 1695 (1–3): 55–72. PMID 15571809. doi:10.1016/j.bbamcr.2004.09.019.
- 1 2 3 4 5 Komander D, Rape M (2012). "The ubiquitin code". Annual Review of Biochemistry. 81: 203–29. PMID 22524316. doi:10.1146/annurev-biochem-060310-170328.
- 1 2 McDowell GS, Philpott A (August 2013). "Non-canonical ubiquitylation: mechanisms and consequences". The International Journal of Biochemistry & Cell Biology. 45 (8): 1833–42. PMID 23732108. doi:10.1016/j.biocel.2013.05.026.
- 1 2 3 4 5 Miranda M, Sorkin A (June 2007). "Regulation of receptors and transporters by ubiquitination: new insights into surprisingly similar mechanisms". Molecular Interventions. 7 (3): 157–67. PMID 17609522. doi:10.1124/mi.7.3.7.
- 1 2 "The Nobel Prize in Chemistry 2004". Nobelprize.org. Retrieved 2010-10-16.
- ↑ "The Nobel Prize in Chemistry 2004: Popular Information". Nobelprize.org. Retrieved 2013-12-14.
- ↑ Ciechanover A, Hod Y, Hershko A (August 2012). "A heat-stable polypeptide component of an ATP-dependent proteolytic system from reticulocytes. 1978". Biochemical and Biophysical Research Communications. 425 (3): 565–70. PMID 22925675. doi:10.1016/j.bbrc.2012.08.025.
- ↑ Wang C, Xi J, Begley TP, Nicholson LK (January 2001). "Solution structure of ThiS and implications for the evolutionary roots of ubiquitin". Nature Structural Biology. 8 (1): 47–51. PMID 11135670. doi:10.1038/83041.
- ↑ Lake MW, Wuebbens MM, Rajagopalan KV, Schindelin H (November 2001). "Mechanism of ubiquitin activation revealed by the structure of a bacterial MoeB-MoaD complex". Nature. 414 (6861): 325–9. PMID 11713534. doi:10.1038/35104586.
- ↑ Hochstrasser M (March 2009). "Origin and function of ubiquitin-like proteins". Nature. 458 (7237): 422–9. PMC 2819001
 . PMID 19325621. doi:10.1038/nature07958.
. PMID 19325621. doi:10.1038/nature07958. - 1 2 Pickart CM (2001). "Mechanisms underlying ubiquitination". Annual Review of Biochemistry. 70: 503–33. PMID 11395416. doi:10.1146/annurev.biochem.70.1.503.
- ↑ Marotti LA, Newitt R, Wang Y, Aebersold R, Dohlman HG (April 2002). "Direct identification of a G protein ubiquitination site by mass spectrometry". Biochemistry. 41 (16): 5067–74. PMID 11955054. doi:10.1021/bi015940q.
- 1 2 Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, Roelofs J, Finley D, Gygi SP (August 2003). "A proteomics approach to understanding protein ubiquitination". Nature Biotechnology. 21 (8): 921–6. PMID 12872131. doi:10.1038/nbt849.
- ↑ Breitschopf K, Bengal E, Ziv T, Admon A, Ciechanover A (October 1998). "A novel site for ubiquitination: the N-terminal residue, and not internal lysines of MyoD, is essential for conjugation and degradation of the protein". The EMBO Journal. 17 (20): 5964–73. PMC 1170923
 . PMID 9774340. doi:10.1093/emboj/17.20.5964.
. PMID 9774340. doi:10.1093/emboj/17.20.5964. - ↑ Bloom J, Amador V, Bartolini F, DeMartino G, Pagano M (October 2003). "Proteasome-mediated degradation of p21 via N-terminal ubiquitinylation". Cell. 115 (1): 71–82. PMID 14532004. doi:10.1016/S0092-8674(03)00755-4.
- 1 2 Scaglione KM, Basrur V, Ashraf NS, Konen JR, Elenitoba-Johnson KS, Todi SV, Paulson HL (June 2013). "The ubiquitin-conjugating enzyme (E2) Ube2w ubiquitinates the N terminus of substrates". The Journal of Biological Chemistry. 288 (26): 18784–8. PMC 3696654
 . PMID 23696636. doi:10.1074/jbc.C113.477596.
. PMID 23696636. doi:10.1074/jbc.C113.477596. - ↑ Sadeh R, Breitschopf K, Bercovich B, Zoabi M, Kravtsova-Ivantsiv Y, Kornitzer D, Schwartz A, Ciechanover A (October 2008). "The N-terminal domain of MyoD is necessary and sufficient for its nuclear localization-dependent degradation by the ubiquitin system". Proceedings of the National Academy of Sciences of the United States of America. 105 (41): 15690–5. PMC 2560994
 . PMID 18836078. doi:10.1073/pnas.0808373105.
. PMID 18836078. doi:10.1073/pnas.0808373105. - ↑ Coulombe P, Rodier G, Bonneil E, Thibault P, Meloche S (July 2004). "N-Terminal ubiquitination of extracellular signal-regulated kinase 3 and p21 directs their degradation by the proteasome". Molecular and Cellular Biology. 24 (14): 6140–50. PMC 434260
 . PMID 15226418. doi:10.1128/MCB.24.14.6140-6150.2004.
. PMID 15226418. doi:10.1128/MCB.24.14.6140-6150.2004. - ↑ Kuo ML, den Besten W, Bertwistle D, Roussel MF, Sherr CJ (August 2004). "N-terminal polyubiquitination and degradation of the Arf tumor suppressor". Genes & Development. 18 (15): 1862–74. PMC 517406
 . PMID 15289458. doi:10.1101/gad.1213904.
. PMID 15289458. doi:10.1101/gad.1213904. - ↑ Ben-Saadon R, Fajerman I, Ziv T, Hellman U, Schwartz AL, Ciechanover A (October 2004). "The tumor suppressor protein p16(INK4a) and the human papillomavirus oncoprotein-58 E7 are naturally occurring lysine-less proteins that are degraded by the ubiquitin system. Direct evidence for ubiquitination at the N-terminal residue". The Journal of Biological Chemistry. 279 (40): 41414–21. PMID 15254040. doi:10.1074/jbc.M407201200.
- ↑ Li H, Okamoto K, Peart MJ, Prives C (February 2009). "Lysine-independent turnover of cyclin G1 can be stabilized by B'alpha subunits of protein phosphatase 2A". Molecular and Cellular Biology. 29 (3): 919–28. PMC 2630686
 . PMID 18981217. doi:10.1128/MCB.00907-08.
. PMID 18981217. doi:10.1128/MCB.00907-08. - ↑ Reinstein E, Scheffner M, Oren M, Ciechanover A, Schwartz A (November 2000). "Degradation of the E7 human papillomavirus oncoprotein by the ubiquitin-proteasome system: targeting via ubiquitination of the N-terminal residue". Oncogene. 19 (51): 5944–50. PMID 11127826. doi:10.1038/sj.onc.1203989.
- ↑ Aviel S, Winberg G, Massucci M, Ciechanover A (August 2000). "Degradation of the epstein-barr virus latent membrane protein 1 (LMP1) by the ubiquitin-proteasome pathway. Targeting via ubiquitination of the N-terminal residue". The Journal of Biological Chemistry. 275 (31): 23491–9. PMID 10807912. doi:10.1074/jbc.M002052200.
- ↑ Ikeda M, Ikeda A, Longnecker R (August 2002). "Lysine-independent ubiquitination of Epstein-Barr virus LMP2A". Virology. 300 (1): 153–9. PMID 12202215. doi:10.1006/viro.2002.1562.
- ↑ Yang J, Hong Y, Wang W, Wu W, Chi Y, Zong H, Kong X, Wei Y, Yun X, Cheng C, Chen K, Gu J (May 2009). "HSP70 protects BCL2L12 and BCL2L12A from N-terminal ubiquitination-mediated proteasomal degradation". FEBS Letters. 583 (9): 1409–14. PMID 19376117. doi:10.1016/j.febslet.2009.04.011.
- ↑ Wang Y, Shao Q, Yu X, Kong W, Hildreth JE, Liu B (May 2011). "N-terminal hemagglutinin tag renders lysine-deficient APOBEC3G resistant to HIV-1 Vif-induced degradation by reduced polyubiquitination". Journal of Virology. 85 (9): 4510–9. PMC 3126286
 . PMID 21345952. doi:10.1128/JVI.01925-10.
. PMID 21345952. doi:10.1128/JVI.01925-10. - ↑ Trausch-Azar JS, Lingbeck J, Ciechanover A, Schwartz AL (July 2004). "Ubiquitin-Proteasome-mediated degradation of Id1 is modulated by MyoD". The Journal of Biological Chemistry. 279 (31): 32614–9. PMID 15163661. doi:10.1074/jbc.M403794200.
- ↑ Trausch-Azar J, Leone TC, Kelly DP, Schwartz AL (December 2010). "Ubiquitin proteasome-dependent degradation of the transcriptional coactivator PGC-1{alpha} via the N-terminal pathway". The Journal of Biological Chemistry. 285 (51): 40192–200. PMC 3001001
 . PMID 20713359. doi:10.1074/jbc.M110.131615.
. PMID 20713359. doi:10.1074/jbc.M110.131615. - ↑ Fajerman I, Schwartz AL, Ciechanover A (February 2004). "Degradation of the Id2 developmental regulator: targeting via N-terminal ubiquitination". Biochemical and Biophysical Research Communications. 314 (2): 505–12. PMID 14733935. doi:10.1016/j.bbrc.2003.12.116.
- 1 2 3 Vosper JM, McDowell GS, Hindley CJ, Fiore-Heriche CS, Kucerova R, Horan I, Philpott A (June 2009). "Ubiquitylation on canonical and non-canonical sites targets the transcription factor neurogenin for ubiquitin-mediated proteolysis". The Journal of Biological Chemistry. 284 (23): 15458–68. PMC 2708843
 . PMID 19336407. doi:10.1074/jbc.M809366200.
. PMID 19336407. doi:10.1074/jbc.M809366200. - 1 2 3 McDowell GS, Kucerova R, Philpott A (October 2010). "Non-canonical ubiquitylation of the proneural protein Ngn2 occurs in both Xenopus embryos and mammalian cells". Biochemical and Biophysical Research Communications. 400 (4): 655–60. PMID 20807509. doi:10.1016/j.bbrc.2010.08.122.
- ↑ Tatham MH, Plechanovová A, Jaffray EG, Salmen H, Hay RT (July 2013). "Ube2W conjugates ubiquitin to α-amino groups of protein N-termini". The Biochemical Journal. 453 (1): 137–45. PMC 3778709
 . PMID 23560854. doi:10.1042/BJ20130244.
. PMID 23560854. doi:10.1042/BJ20130244. - ↑ Vittal V, Shi L, Wenzel DM, Scaglione KM, Duncan ED, Basrur V, Elenitoba-Johnson KS, Baker D, Paulson HL, Brzovic PS, Klevit RE (January 2015). "Intrinsic disorder drives N-terminal ubiquitination by Ube2w". Nature Chemical Biology. 11 (1): 83–9. PMC 4270946
 . PMID 25436519. doi:10.1038/nchembio.1700.
. PMID 25436519. doi:10.1038/nchembio.1700. - ↑ Johnson ES, Ma PC, Ota IM, Varshavsky A (July 1995). "A proteolytic pathway that recognizes ubiquitin as a degradation signal". The Journal of Biological Chemistry. 270 (29): 17442–56. PMID 7615550. doi:10.1074/jbc.270.29.17442.
- 1 2 Kirisako T, Kamei K, Murata S, Kato M, Fukumoto H, Kanie M, Sano S, Tokunaga F, Tanaka K, Iwai K (October 2006). "A ubiquitin ligase complex assembles linear polyubiquitin chains". The EMBO Journal. 25 (20): 4877–87. PMC 1618115
 . PMID 17006537. doi:10.1038/sj.emboj.7601360.
. PMID 17006537. doi:10.1038/sj.emboj.7601360. - 1 2 Wang X, Herr RA, Chua WJ, Lybarger L, Wiertz EJ, Hansen TH (May 2007). "Ubiquitination of serine, threonine, or lysine residues on the cytoplasmic tail can induce ERAD of MHC-I by viral E3 ligase mK3". The Journal of Cell Biology. 177 (4): 613–24. PMC 2064207
 . PMID 17502423. doi:10.1083/jcb.200611063.
. PMID 17502423. doi:10.1083/jcb.200611063. - ↑ Cadwell K, Coscoy L (July 2005). "Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase". Science. 309 (5731): 127–30. PMID 15994556. doi:10.1126/science.1110340.
- ↑ Cadwell K, Coscoy L (April 2008). "The specificities of Kaposi's sarcoma-associated herpesvirus-encoded E3 ubiquitin ligases are determined by the positions of lysine or cysteine residues within the intracytoplasmic domains of their targets". Journal of Virology. 82 (8): 4184–9. PMC 2293015
 . PMID 18272573. doi:10.1128/JVI.02264-07.
. PMID 18272573. doi:10.1128/JVI.02264-07. - ↑ Williams C, van den Berg M, Sprenger RR, Distel B (August 2007). "A conserved cysteine is essential for Pex4p-dependent ubiquitination of the peroxisomal import receptor Pex5p". The Journal of Biological Chemistry. 282 (31): 22534–43. PMID 17550898. doi:10.1074/jbc.M702038200.
- ↑ Carvalho AF, Pinto MP, Grou CP, Alencastre IS, Fransen M, Sá-Miranda C, Azevedo JE (October 2007). "Ubiquitination of mammalian Pex5p, the peroxisomal import receptor". The Journal of Biological Chemistry. 282 (43): 31267–72. PMID 17726030. doi:10.1074/jbc.M706325200.
- ↑ Léon S, Subramani S (March 2007). "A conserved cysteine residue of Pichia pastoris Pex20p is essential for its recycling from the peroxisome to the cytosol". The Journal of Biological Chemistry. 282 (10): 7424–30. PMC 3682499
 . PMID 17209040. doi:10.1074/jbc.M611627200.
. PMID 17209040. doi:10.1074/jbc.M611627200. - 1 2 Tait SW, de Vries E, Maas C, Keller AM, D'Santos CS, Borst J (December 2007). "Apoptosis induction by Bid requires unconventional ubiquitination and degradation of its N-terminal fragment". The Journal of Cell Biology. 179 (7): 1453–66. PMC 2373500
 . PMID 18166654. doi:10.1083/jcb.200707063.
. PMID 18166654. doi:10.1083/jcb.200707063. - 1 2 Roark R, Itzhaki L, Philpott A (December 2012). "Complex regulation controls Neurogenin3 proteolysis". Biology Open. 1 (12): 1264–72. PMC 3522888
 . PMID 23259061. doi:10.1242/bio.20121750.
. PMID 23259061. doi:10.1242/bio.20121750. - ↑ Magadán JG, Pérez-Victoria FJ, Sougrat R, Ye Y, Strebel K, Bonifacino JS (April 2010). "Multilayered mechanism of CD4 downregulation by HIV-1 Vpu involving distinct ER retention and ERAD targeting steps". PLoS Pathogens. 6 (4): e1000869. PMC 2861688
 . PMID 20442859. doi:10.1371/journal.ppat.1000869.
. PMID 20442859. doi:10.1371/journal.ppat.1000869. - ↑ Tokarev AA, Munguia J, Guatelli JC (January 2011). "Serine-threonine ubiquitination mediates downregulation of BST-2/tetherin and relief of restricted virion release by HIV-1 Vpu". Journal of Virology. 85 (1): 51–63. PMC 3014196
 . PMID 20980512. doi:10.1128/JVI.01795-10.
. PMID 20980512. doi:10.1128/JVI.01795-10. - ↑ Ishikura S, Weissman AM, Bonifacino JS (July 2010). "Serine residues in the cytosolic tail of the T-cell antigen receptor alpha-chain mediate ubiquitination and endoplasmic reticulum-associated degradation of the unassembled protein". The Journal of Biological Chemistry. 285 (31): 23916–24. PMC 2911338
 . PMID 20519503. doi:10.1074/jbc.M110.127936.
. PMID 20519503. doi:10.1074/jbc.M110.127936. - ↑ Shimizu Y, Okuda-Shimizu Y, Hendershot LM (December 2010). "Ubiquitylation of an ERAD substrate occurs on multiple types of amino acids". Molecular Cell. 40 (6): 917–26. PMC 3031134
 . PMID 21172657. doi:10.1016/j.molcel.2010.11.033.
. PMID 21172657. doi:10.1016/j.molcel.2010.11.033. - ↑ Dikic I, Robertson M (March 2012). "Ubiquitin ligases and beyond". BMC Biology. 10: 22. PMC 3305657
 . PMID 22420755. doi:10.1186/1741-7007-10-22.
. PMID 22420755. doi:10.1186/1741-7007-10-22. - ↑ Schulman BA, Harper JW (May 2009). "Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways". Nature Reviews Molecular Cell Biology. 10 (5): 319–31. PMC 2712597
 . PMID 19352404. doi:10.1038/nrm2673.
. PMID 19352404. doi:10.1038/nrm2673. - ↑ Groettrup M, Pelzer C, Schmidtke G, Hofmann K (May 2008). "Activating the ubiquitin family: UBA6 challenges the field". Trends in Biochemical Sciences. 33 (5): 230–7. PMID 18353650. doi:10.1016/j.tibs.2008.01.005.
- ↑ van Wijk SJ, Timmers HT (April 2010). "The family of ubiquitin-conjugating enzymes (E2s): deciding between life and death of proteins". FASEB Journal. 24 (4): 981–93. PMID 19940261. doi:10.1096/fj.09-136259.
- ↑ Metzger MB, Hristova VA, Weissman AM (February 2012). "HECT and RING finger families of E3 ubiquitin ligases at a glance". Journal of Cell Science. 125 (Pt 3): 531–7. PMC 3381717
 . PMID 22389392. doi:10.1242/jcs.091777.
. PMID 22389392. doi:10.1242/jcs.091777. - ↑ Skaar JR, Pagano M (December 2009). "Control of cell growth by the SCF and APC/C ubiquitin ligases". Current Opinion in Cell Biology. 21 (6): 816–24. PMC 2805079
 . PMID 19775879. doi:10.1016/j.ceb.2009.08.004.
. PMID 19775879. doi:10.1016/j.ceb.2009.08.004. - ↑ Kerscher O, Felberbaum R, Hochstrasser M (2006). "Modification of proteins by ubiquitin and ubiquitin-like proteins". Annual Review of Cell and Developmental Biology. 22: 159–80. PMID 16753028. doi:10.1146/annurev.cellbio.22.010605.093503.
- ↑ Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S (March 1999). "A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly". Cell. 96 (5): 635–44. PMID 10089879. doi:10.1016/S0092-8674(00)80574-7.
- ↑ Lai Z, Ferry KV, Diamond MA, Wee KE, Kim YB, Ma J, Yang T, Benfield PA, Copeland RA, Auger KR (August 2001). "Human mdm2 mediates multiple mono-ubiquitination of p53 by a mechanism requiring enzyme isomerization". The Journal of Biological Chemistry. 276 (33): 31357–67. PMID 11397792. doi:10.1074/jbc.M011517200.
- ↑ Grossman SR, Deato ME, Brignone C, Chan HM, Kung AL, Tagami H, Nakatani Y, Livingston DM (April 2003). "Polyubiquitination of p53 by a ubiquitin ligase activity of p300". Science. 300 (5617): 342–4. PMID 12690203. doi:10.1126/science.1080386.
- ↑ Shi D, Pop MS, Kulikov R, Love IM, Kung AL, Kung A, Grossman SR (September 2009). "CBP and p300 are cytoplasmic E4 polyubiquitin ligases for p53". Proceedings of the National Academy of Sciences of the United States of America. 106 (38): 16275–80. PMC 2752525
 . PMID 19805293. doi:10.1073/pnas.0904305106.
. PMID 19805293. doi:10.1073/pnas.0904305106. - 1 2 3 4 5 Komander D (October 2009). "The emerging complexity of protein ubiquitination". Biochemical Society Transactions. 37 (Pt 5): 937–53. PMID 19754430. doi:10.1042/BST0370937.
- 1 2 3 Ikeda F, Dikic I (June 2008). "Atypical ubiquitin chains: new molecular signals. 'Protein Modifications: Beyond the Usual Suspects' review series". EMBO Reports. 9 (6): 536–42. PMC 2427391
 . PMID 18516089. doi:10.1038/embor.2008.93.
. PMID 18516089. doi:10.1038/embor.2008.93. - ↑ Xu P, Peng J (May 2008). "Characterization of polyubiquitin chain structure by middle-down mass spectrometry". Analytical Chemistry. 80 (9): 3438–44. PMC 2663523
 . PMID 18351785. doi:10.1021/ac800016w.
. PMID 18351785. doi:10.1021/ac800016w. - 1 2 Hicke L (March 2001). "Protein regulation by monoubiquitin". Nature Reviews Molecular Cell Biology. 2 (3): 195–201. PMID 11265249. doi:10.1038/35056583.
- ↑ Lecker SH, Goldberg AL, Mitch WE (July 2006). "Protein degradation by the ubiquitin-proteasome pathway in normal and disease states". Journal of the American Society of Nephrology. 17 (7): 1807–19. PMID 16738015. doi:10.1681/ASN.2006010083.
- 1 2 Kravtsova-Ivantsiv Y, Ciechanover A (February 2012). "Non-canonical ubiquitin-based signals for proteasomal degradation". Journal of Cell Science. 125 (Pt 3): 539–48. PMID 22389393. doi:10.1242/jcs.093567.
- ↑ Nathan JA, Kim HT, Ting L, Gygi SP, Goldberg AL (February 2013). "Why do cellular proteins linked to K63-polyubiquitin chains not associate with proteasomes?". The EMBO Journal. 32 (4): 552–65. PMC 3579138
 . PMID 23314748. doi:10.1038/emboj.2012.354.
. PMID 23314748. doi:10.1038/emboj.2012.354. - ↑ Bache KG, Raiborg C, Mehlum A, Stenmark H (April 2003). "STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes". The Journal of Biological Chemistry. 278 (14): 12513–21. PMID 12551915. doi:10.1074/jbc.M210843200.
- 1 2 Zhao S, Ulrich HD (April 2010). "Distinct consequences of posttranslational modification by linear versus K63-linked polyubiquitin chains". Proceedings of the National Academy of Sciences of the United States of America. 107 (17): 7704–9. PMC 2867854
 . PMID 20385835. doi:10.1073/pnas.0908764107.
. PMID 20385835. doi:10.1073/pnas.0908764107. - ↑ Kim HT, Kim KP, Lledias F, Kisselev AF, Scaglione KM, Skowyra D, Gygi SP, Goldberg AL (June 2007). "Certain pairs of ubiquitin-conjugating enzymes (E2s) and ubiquitin-protein ligases (E3s) synthesize nondegradable forked ubiquitin chains containing all possible isopeptide linkages". The Journal of Biological Chemistry. 282 (24): 17375–86. PMID 17426036. doi:10.1074/jbc.M609659200.
- ↑ Michel MA, Elliott PR, Swatek KN, Simicek M, Pruneda JN, Wagstaff JL, Freund SM, Komander D (April 2015). "Assembly and specific recognition of k29- and k33-linked polyubiquitin". Molecular Cell. 58 (1): 95–109. PMC 4386031
 . PMID 25752577. doi:10.1016/j.molcel.2015.01.042.
. PMID 25752577. doi:10.1016/j.molcel.2015.01.042. - 1 2 "Ubiquitin Proteasome Pathway Overview". Archived from the original on 2008-03-30. Retrieved 2008-04-30.
- ↑ Shaheen M, Shanmugam I, Hromas R (August 2010). "The Role of PCNA Posttranslational Modifications in Translesion Synthesis". Journal of Nucleic Acids. 2010: 1–8. PMC 2935186
 . PMID 20847899. doi:10.4061/2010/761217.
. PMID 20847899. doi:10.4061/2010/761217. - ↑ Jackson SP, Durocher D (March 2013). "Regulation of DNA damage responses by ubiquitin and SUMO". Molecular Cell. 49 (5): 795–807. PMID 23416108. doi:10.1016/j.molcel.2013.01.017.
- ↑ Campbell SJ, Edwards RA, Leung CC, Neculai D, Hodge CD, Dhe-Paganon S, Glover JN (July 2012). "Molecular insights into the function of RING finger (RNF)-containing proteins hRNF8 and hRNF168 in Ubc13/Mms2-dependent ubiquitylation". The Journal of Biological Chemistry. 287 (28): 23900–10. PMC 3390666
 . PMID 22589545. doi:10.1074/jbc.M112.359653.
. PMID 22589545. doi:10.1074/jbc.M112.359653. - ↑ Ikura T, Tashiro S, Kakino A, Shima H, Jacob N, Amunugama R, Yoder K, Izumi S, Kuraoka I, Tanaka K, Kimura H, Ikura M, Nishikubo S, Ito T, Muto A, Miyagawa K, Takeda S, Fishel R, Igarashi K, Kamiya K (October 2007). "DNA damage-dependent acetylation and ubiquitination of H2AX enhances chromatin dynamics". Molecular and Cellular Biology. 27 (20): 7028–40. PMC 2168918
 . PMID 17709392. doi:10.1128/MCB.00579-07.
. PMID 17709392. doi:10.1128/MCB.00579-07. - ↑ Kim H, Chen J, Yu X (May 2007). "Ubiquitin-binding protein RAP80 mediates BRCA1-dependent DNA damage response". Science. 316 (5828): 1202–5. PMID 17525342. doi:10.1126/science.1139621.
- ↑ Hofmann K (April 2009). "Ubiquitin-binding domains and their role in the DNA damage response". DNA Repair. 8 (4): 544–56. PMID 19213613. doi:10.1016/j.dnarep.2009.01.003.
- ↑ Hammond-Martel I, Yu H, Affar el B (February 2012). "Roles of ubiquitin signaling in transcription regulation". Cellular Signalling. 24 (2): 410–21. PMID 22033037. doi:10.1016/j.cellsig.2011.10.009.
- ↑ Reyes-Turcu FE, Ventii KH, Wilkinson KD (2009). "Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes". Annual Review of Biochemistry. 78: 363–97. PMC 2734102
 . PMID 19489724. doi:10.1146/annurev.biochem.78.082307.091526.
. PMID 19489724. doi:10.1146/annurev.biochem.78.082307.091526. - ↑ Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R (December 2005). "A genomic and functional inventory of deubiquitinating enzymes". Cell. 123 (5): 773–86. PMID 16325574. doi:10.1016/j.cell.2005.11.007.
- 1 2 Hicke L, Schubert HL, Hill CP (August 2005). "Ubiquitin-binding domains". Nature Reviews Molecular Cell Biology. 6 (8): 610–21. PMID 16064137. doi:10.1038/nrm1701.
- ↑ Husnjak K, Dikic I (2012-01-01). "Ubiquitin-binding proteins: decoders of ubiquitin-mediated cellular functions". Annual Review of Biochemistry. 81: 291–322. PMID 22482907. doi:10.1146/annurev-biochem-051810-094654.
- ↑ "UBQLN1 ubiquilin 1 [ Homo sapiens ]". Gene. National Center for Biotechnology Information. Retrieved 9 May 2012.
- 1 2 Stieren ES, El Ayadi A, Xiao Y, Siller E, Landsverk ML, Oberhauser AF, Barral JM, Boehning D (October 2011). "Ubiquilin-1 is a molecular chaperone for the amyloid precursor protein". The Journal of Biological Chemistry. 286 (41): 35689–98. PMC 3195644
 . PMID 21852239. doi:10.1074/jbc.M111.243147. Lay summary – Science Daily.
. PMID 21852239. doi:10.1074/jbc.M111.243147. Lay summary – Science Daily. - ↑ Huber C, Dias-Santagata D, Glaser A, O'Sullivan J, Brauner R, Wu K, Xu X, Pearce K, Wang R, Uzielli ML, Dagoneau N, Chemaitilly W, Superti-Furga A, Dos Santos H, Mégarbané A, Morin G, Gillessen-Kaesbach G, Hennekam R, Van der Burgt I, Black GC, Clayton PE, Read A, Le Merrer M, Scambler PJ, Munnich A, Pan ZQ, Winter R, Cormier-Daire V (October 2005). "Identification of mutations in CUL7 in 3-M syndrome". Nature Genetics. 37 (10): 1119–24. PMID 16142236. doi:10.1038/ng1628.
- 1 2 3 Nguyen LK, Kolch W, Kholodenko BN (July 2013). "When ubiquitination meets phosphorylation: a systems biology perspective of EGFR/MAPK signalling". Cell Communication and Signaling. 11: 52. PMC 3734146
 . PMID 23902637. doi:10.1186/1478-811X-11-52.
. PMID 23902637. doi:10.1186/1478-811X-11-52. - ↑ Sorkin A, Goh LK (October 2008). "Endocytosis and intracellular trafficking of ErbBs". Experimental Cell Research. 314 (17): 3093–106. PMC 2605728
 . PMID 18793634. doi:10.1016/j.yexcr.2008.07.029.
. PMID 18793634. doi:10.1016/j.yexcr.2008.07.029. - ↑ Nguyen LK, Muñoz-García J, Maccario H, Ciechanover A, Kolch W, Kholodenko BN (December 2011). "Switches, excitable responses and oscillations in the Ring1B/Bmi1 ubiquitination system". PLoS Computational Biology. 7 (12): e1002317. PMC 3240587
 . PMID 22194680. doi:10.1371/journal.pcbi.1002317.
. PMID 22194680. doi:10.1371/journal.pcbi.1002317. - ↑ Zhou W, Wang X, Rosenfeld MG (January 2009). "Histone H2A ubiquitination in transcriptional regulation and DNA damage repair". The International Journal of Biochemistry & Cell Biology. 41 (1): 12–5. PMID 18929679. doi:10.1016/j.biocel.2008.09.016.
- ↑ Dou QP, Li B (August 1999). "Proteasome inhibitors as potential novel anticancer agents". Drug Resistance Updates. 2 (4): 215–223. PMID 11504494. doi:10.1054/drup.1999.0095.
- ↑ Vries EG, Verweij J (2000). "Clinical cancer research 2000: new agents and therapies". Drug Resistance Updates : Reviews and Commentaries in Antimicrobial and Anticancer Chemotherapy. 3 (4): 197–201. PMID 11498385. doi:10.1054/drup.2000.0153.
- 1 2 3 Pray TR, Parlati F, Huang J, Wong BR, Payan DG, Bennett MK, Issakani SD, Molineaux S, Demo SD (December 2002). "Cell cycle regulatory E3 ubiquitin ligases as anticancer targets". Drug Resistance Updates. 5 (6): 249–58. PMID 12531181. doi:10.1016/s1368-7646(02)00121-8.
- 1 2 Clifford SC, Cockman ME, Smallwood AC, Mole DR, Woodward ER, Maxwell PH, Ratcliffe PJ, Maher ER (2001). "Contrasting effects on HIF-1alpha regulation by disease-causing pVHL mutations correlate with patterns of tumourigenesis in von Hippel-Lindau disease". Human Molecular Genetics. 10 (10): 1029–38. PMID 11331613. doi:10.1093/hmg/10.10.1029.
- 1 2 Sparks AB, Morin PJ, Vogelstein B, Kinzler KW (March 1998). "Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer". Cancer Research. 58 (6): 1130–4. PMID 9515795.
- 1 2 Scheffner M, Huibregtse JM, Vierstra RD, Howley PM (November 1993). "The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53". Cell. 75 (3): 495–505. PMID 8221889. doi:10.1016/0092-8674(93)90384-3.
- 1 2 Momand J, Jung D, Wilczynski S, Niland J (August 1998). "The MDM2 gene amplification database". Nucleic Acids Research. 26 (15): 3453–9. PMC 147746
 . PMID 9671804. doi:10.1093/nar/26.15.3453.
. PMID 9671804. doi:10.1093/nar/26.15.3453. - 1 2 Hashizume R, Fukuda M, Maeda I, Nishikawa H, Oyake D, Yabuki Y, Ogata H, Ohta T (May 2001). "The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation". The Journal of Biological Chemistry. 276 (18): 14537–40. PMID 11278247. doi:10.1074/jbc.C000881200.
- 1 2 Zhu CQ, Blackhall FH, Pintilie M, Iyengar P, Liu N, Ho J, Chomiak T, Lau D, Winton T, Shepherd FA, Tsao MS (2004). "Skp2 gene copy number aberrations are common in non-small cell lung carcinoma, and its overexpression in tumors with ras mutation is a poor prognostic marker". Clinical Cancer Research : an Official Journal of the American Association for Cancer Research. 10 (6): 1984–91. PMID 15041716. doi:10.1158/1078-0432.ccr-03-0470.
- 1 2 Schmidt MH, Furnari FB, Cavenee WK, Bögler O (May 2003). "Epidermal growth factor receptor signaling intensity determines intracellular protein interactions, ubiquitination, and internalization". Proceedings of the National Academy of Sciences of the United States of America. 100 (11): 6505–10. PMC 164476
 . PMID 12734385. doi:10.1073/pnas.1031790100.
. PMID 12734385. doi:10.1073/pnas.1031790100. - 1 2 Knuutila S, Aalto Y, Autio K, Björkqvist AM, El-Rifai W, Hemmer S, Huhta T, Kettunen E, Kiuru-Kuhlefelt S, Larramendy ML, Lushnikova T, Monni O, Pere H, Tapper J, Tarkkanen M, Varis A, Wasenius VM, Wolf M, Zhu Y (September 1999). "DNA copy number losses in human neoplasms". The American Journal of Pathology. 155 (3): 683–94. PMC 1866903
 . PMID 10487825. doi:10.1016/S0002-9440(10)65166-8.
. PMID 10487825. doi:10.1016/S0002-9440(10)65166-8. - 1 2 3 4 5 6 7 Mani A, Gelmann EP (July 2005). "The ubiquitin-proteasome pathway and its role in cancer". Journal of Clinical Oncology. 23 (21): 4776–89. PMID 16034054. doi:10.1200/JCO.2005.05.081.
- 1 2 Nalepa G, Wade Harper J (May 2003). "Therapeutic anti-cancer targets upstream of the proteasome". Cancer Treatment Reviews. 29 Suppl 1: 49–57. PMID 12738243. doi:10.1016/s0305-7372(03)00083-5.
- ↑ Witowsky JA, Johnson GL (January 2003). "Ubiquitylation of MEKK1 inhibits its phosphorylation of MKK1 and MKK4 and activation of the ERK1/2 and JNK pathways". The Journal of Biological Chemistry. 278 (3): 1403–6. PMID 12456688. doi:10.1074/jbc.C200616200.
- 1 2 3 Kobashigawa Y, Tomitaka A, Kumeta H, Noda NN, Yamaguchi M, Inagaki F (December 2011). "Autoinhibition and phosphorylation-induced activation mechanisms of human cancer and autoimmune disease-related E3 protein Cbl-b". Proceedings of the National Academy of Sciences of the United States of America. 108 (51): 20579–84. PMC 3251137
 . PMID 22158902. doi:10.1073/pnas.1110712108.
. PMID 22158902. doi:10.1073/pnas.1110712108. - ↑ Niemeyer CM, Kang MW, Shin DH, Furlan I, Erlacher M, Bunin NJ, Bunda S, Finklestein JZ, Sakamoto KM, Gorr TA, Mehta P, Schmid I, Kropshofer G, Corbacioglu S, Lang PJ, Klein C, Schlegel PG, Heinzmann A, Schneider M, Starý J, van den Heuvel-Eibrink MM, Hasle H, Locatelli F, Sakai D, Archambeault S, Chen L, Russell RC, Sybingco SS, Ohh M, Braun BS, Flotho C, Loh ML (September 2010). "Germline CBL mutations cause developmental abnormalities and predispose to juvenile myelomonocytic leukemia". Nature Genetics. 42 (9): 794–800. PMC 4297285
 . PMID 20694012. doi:10.1038/ng.641.
. PMID 20694012. doi:10.1038/ng.641. - ↑ Kales SC, Ryan PE, Nau MM, Lipkowitz S (June 2010). "Cbl and human myeloid neoplasms: the Cbl oncogene comes of age". Cancer Research. 70 (12): 4789–94. PMC 2888780
 . PMID 20501843. doi:10.1158/0008-5472.CAN-10-0610.
. PMID 20501843. doi:10.1158/0008-5472.CAN-10-0610. - ↑ Yen HC, Elledge SJ (2008). "Identification of SCF ubiquitin ligase substrates by global protein stability profiling". Science. 322 (5903): 923–9. PMID 18988848. doi:10.1126/science.1160462.
- ↑ Downes BP, Saracco SA, Lee SS, Crowell DN, Vierstra RD (September 2006). "MUBs, a family of ubiquitin-fold proteins that are plasma membrane-anchored by prenylation". The Journal of Biological Chemistry. 281 (37): 27145–57. PMID 16831869. doi:10.1074/jbc.M602283200.
- ↑ Welchman RL, Gordon C, Mayer RJ (August 2005). "Ubiquitin and ubiquitin-like proteins as multifunctional signals". Nature Reviews Molecular Cell Biology. 6 (8): 599–609. PMID 16064136. doi:10.1038/nrm1700.
- ↑ Grabbe C, Dikic I (April 2009). "Functional roles of ubiquitin-like domain (ULD) and ubiquitin-binding domain (UBD) containing proteins". Chemical Reviews. 109 (4): 1481–94. PMID 19253967. doi:10.1021/cr800413p.
- ↑ Sutovsky P, Moreno RD, Ramalho-Santos J, Dominko T, Simerly C, Schatten G (August 2000). "Ubiquitinated sperm mitochondria, selective proteolysis, and the regulation of mitochondrial inheritance in mammalian embryos". Biology of Reproduction. 63 (2): 582–90. PMID 10906068. doi:10.1095/biolreprod63.2.582.
- 1 2 Lehmann, G; Udasin, R.G.; Livneh, I.; Ciechanover, A. (2017). "Identification of UBact, a ubiquitin-like protein, along with other homologous components of a conjugation system and the proteasome in different gram-negative bacteria.". Biochem Biophys Res Commun. 483 (3): 946–950. PMID 28087277. doi:10.1016/j.bbrc.2017.01.037.
- ↑ Tung CW, Ho SY (July 2008). "Computational identification of ubiquitylation sites from protein sequences". BMC Bioinformatics. 9: 310. PMC 2488362
 . PMID 18625080. doi:10.1186/1471-2105-9-310.
. PMID 18625080. doi:10.1186/1471-2105-9-310. - ↑ Radivojac P, Vacic V, Haynes C, Cocklin RR, Mohan A, Heyen JW, Goebl MG, Iakoucheva LM (February 2010). "Identification, analysis, and prediction of protein ubiquitination sites". Proteins. 78 (2): 365–80. PMC 3006176
 . PMID 19722269. doi:10.1002/prot.22555.
. PMID 19722269. doi:10.1002/prot.22555. - ↑ Chen Z, Chen YZ, Wang XF, Wang C, Yan RX, Zhang Z (2011). Fraternali F, ed. "Prediction of ubiquitination sites by using the composition of k-spaced amino acid pairs". PloS One. 6 (7): e22930. PMC 3146527
 . PMID 21829559. doi:10.1371/journal.pone.0022930.
. PMID 21829559. doi:10.1371/journal.pone.0022930.
External links
- GeneReviews/NCBI/NIH/UW entry on Angelman syndrome
- OMIM entries on Angelman syndrome
- UniProt entry for ubiquitin
- "7.340 Ubiquitination: The Proteasome and Human Disease". MIT OpenCourseWare. 2004. Notes from MIT course.
- Ubiquitin at the US National Library of Medicine Medical Subject Headings (MeSH)