Tungsten(IV) chloride
 | |
| Names | |
|---|---|
| Other names
tungsten tetrachloride | |
| Identifiers | |
| ECHA InfoCard | 100.157.353 |
| Properties | |
| Cl4W | |
| Molar mass | 325.65 g·mol−1 |
| Appearance | black solid |
| Density | 4.62 g·cm−3 |
| Melting point | 450 °C (842 °F; 723 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tungsten(IV)-chloride is an inorganic compound with the formula WCl4. It is a diamagnetic black solid. The compound is of interest in research as one of a handful of binary tungsten chlorides.
Structure and preparation
WCl4 is usually prepared by reduction tungsten hexachloride. Many reductants have been reported, including red phosphorus, tungsten hexacarbonyl, gallium, tin, and antimony. The latter is reported to be optimal:[1]
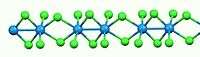
Like most binary metal halides, WCl4 is polymeric. It consists of a linear chains of tungsten atoms each in octahedral geometry. Of six chloride ligands attached to each W center, four are bridging ligands. The W-W separations are alternatingly bonding (2.688 Å) and nonbonding (3.787 Å).
References
- ↑ Y. Zhou, V. Kolesnichenko, L. Messerle "Crystalline and Amorphous Forms of Tungsten Tetrachloride" Inorg. Synth. 2014, vol. 36, pages 30–34. doi: 10.1002/9781118744994.ch6
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.