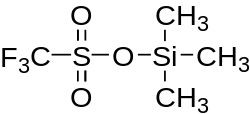
Trimethylsilyl trifluoromethanesulfonate
 | |
| Names | |
|---|---|
| IUPAC name
trimethylsilyl trifluoromethanesulfonate | |
| Other names
TMSOTf Trimethylsilyl triflate TMS triflate Trifluoromethanesulfonic acid trimethylsilyl ester | |
| Identifiers | |
| 3D model (JSmol) |
|
| ECHA InfoCard | 100.044.136 |
| PubChem CID |
|
| |
| Properties | |
| C4H9F3O3SSi | |
| Molar mass | 222.25 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Trimethylsilyl trifluoromethanesulfonate is a trifluoromethanesulfonate derivate with a trimethylsilyl R-group. It has similar reactivity to trimethylsilyl chloride, and is also used often in organic synthesis.
Examples of use
The stereoselective synthesis of seven benzylated proanthocyanidin trimers (epicatechin-(4β-8)-epicatechin-(4β-8)-epicatechin trimer (procyanidin C1), catechin-(4α-8)-catechin-(4α-8)-catechin trimer (procyanidin C2), epicatechin-(4β-8)-epicatechin-(4β-8)-catechin trimer and epicatechin-(4β-8)-catechin-(4α-8)-epicatechin trimer derivatives) can be achieved with TMSOTf-catalyzed condensation reaction, in excellent yields. Deprotection of (+)-catechin and (−)-epicatechin trimers derivatives gives four natural procyanidin trimers in good yields.[1]
It has been used in Takahashi Taxol total synthesis or for chemical glycosylation reactions.
See also
References
- ↑ Efficient Stereoselective Synthesis of Proanthocyanidin Trimers with TMSOTf-Catalyzed Intermolecular Condensation. Akiko Saito, Akira Tanaka, Makoto Ubukata and Noriyuki Nakajima, Synlett, 2004, volume 6, pages 1069-1073, doi:10.1055/s-2004-822905