Trimethylenemethane
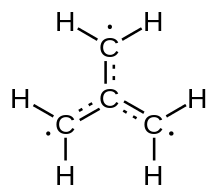
Trimethylenemethane (often abbreviated TMM) is a chemical compound with formula C
4H
6. It is a neutral free molecule with two unsatisfied valence bonds, and is therefore a highly reactive free radical. Formally, it can be viewed as an isobutylene molecule C
4H
8 with two hydrogen atoms removed from the terminal methyl groups.
Trimethylenemethane was first conjectured to be an intermediate in some chemical reactions. It was observed in 1966 by Paul Dowd at very low temperature.[1]
Structure
The unusual electronic structure of Trimethylenemethane was recognized by Moffit and Coulson in 1948.[2][3] It is a neutral four-carbon molecule containing four pi bonds. When trapped in a solid matrix at about 90 K, the six hydrogen atoms of the molecule are undistinguishable by spectroscopy. Thus, it must be described either as zwitterion, or as the simplest conjugated hydrocarbon that cannot be given a Kekulé structure. It can be described as the superposition of three states:
 |
 |
 |
In 1966 Paul Dowd determined with electron spin resonance that this molecule has a triplet ground state (3
A
2'/3
B
2), and is therefore a diradical in the stricter sense of the term. Calculations predict that in this state the molecule is planar with three-fold rotational symmetry, with approximate bond lengths 1.40 Å (C–C) and 1.08 Å (C–H). The H–C–H angle in each methylene is about 121 degrees.[2]
The molecule also exists as three singlet states with slightly higher energy. According to theoretical calculations, the first one, 11
A
1 (1.17 eV above ground), is a closed shell diradical with flat geometry and fully degenerate threefold (D3h) symmetry. The second one, 11
B
2 (also at 1.17 eV), is an open-shell radical with a D3h-symmetric equilibrium between three equal geometries; each has a longer C–C bond (1.48 Å) and two shorter ones (1.38 Å), and is flat and bilaterally symmetric except that the longer methylene is twisted 79 degrees out of the plane (C2 symmetry). The third singlet state, 21
A
1/1
A
1' (3.88 eV), is also a D3h-symmetric equilibrium of three geometries; each is planar with one shorter C–C bond and two longer ones (C2ν symmetry).[2]
The next higher energy states are degenerate triplets, 13
A
1 and 23
B
2 (4.61 eV), with one excited electron; and a quintet state, 5
B
2 (7.17 eV), with the p orbitals occupied by single electrons and D3h symmetry.[2]
Preparation
Trimethylenemethane was first obtained from photolysis of the diazo compound 4-methylene-Δ1-pyrazoline with expulsion of nitrogen, in a frozen dilute glassy solution at −196 C.[1]
It was also obtained from photolysis of 3-methylenecyclobutanone, both in cold solution and in the form of a single crystal, with expulsion of carbon monoxide. In both cases, trimethylenemethane was detected by electron spin resonance spectroscopy.[1]

Trimethylenemethane has been obtained also by reacting potassium with isobutylene diiodide (IH
2C)2C=CH
2 in the gas phase. However the product quickly dimerizes to yield 1,4-dimethylenecyclohexane, and also 2-methylpropene by abstracting two hydrogen atoms from other molecules (hydrocarbon or potassium hydride).[4]
Reactions
The molecule is an isomer of methylenecyclopropane, and can convert to it, but only after being raised to the singlet state.
Organometallic chemistry
A number of organometallic complexes have been prepared, starting with Fe(C
4H
6)(CO)3), which was obtained by the ring-opening of methylenecyclopropane with diiron nonacarbonyl (Fe
2(CO)9.[1] The same complex was prepared by the salt metathesis reaction of disodium tetracarbonylferrate (Na
2Fe(CO)4) with 3-chloro-2-(chloromethyl)propene.[5] Related reactions give M(TMM)(CO)3 (M = Cr, Mo). The reaction leading to (TMM)Mo(CO)4 also gives Mo(C
8H
12)(CO)3 containing a dimerized TMM ligand.[5]
Complexes of TMM derivatives have been studied, for their potential in organic synthesis, specifically in the trimethylenemethane cycloaddition reaction. One example is a palladium-catalyzed [3+2] cycloaddition of trimethylenemethane.[6]
References
- 1 2 3 4 Paul Dowd (1972), Trimethylenemethane. Accounts of Chemical Research, volume 5, issue 7, pages 242–248. doi:10.1021/ar50055a003
- 1 2 3 4 Lyudmila V. Slipchenko and Anna I. Krylov (2003), Electronic structure of the trimethylenemethane diradical in its ground and electronically excited states: Bonding, equilibrium geometries, and vibrational frequencies. Journal of Chemical Physics volume 118, issue 15, 6874-6883 doi:10.1063/1.1561052
- ↑ C. A. Coulson (1948), Journal de Chimie Physique et de Physico-Chimie Biologique, volume 45, page 243. Cited by Slipchenko and Krylov (2003).
- ↑ Philip S. Skell , Robert G. Doerr (1967) Trimethylenemethane. Journal of the American Chemical Society, volume 89, issue 18, pages 4688–4692. doi:10.1021/ja00994a020
- 1 2 J. S. Ward and R. Pettit (1970). "Trimethylenemethane complexes of iron, molybdenum, and chromium". Journal of the Chemical Society Series D: 1419–1420doi=10.1039/C29700001419.
- ↑ New conjunctive reagents. 2-Acetoxymethyl-3-allyltrimethylsilane for methylenecyclopentane annulations catalyzed by palladium(0) Barry M. Trost, Dominic M. T. Chan J. Am. Chem. Soc., 1979, 101 (21), pp 6429–6432 doi:10.1021/ja00515a046