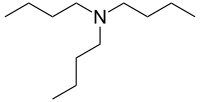
Tributylamine
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N,N-Dibutylbutan-1-amine | |
| Other names
(Tributyl)amine (The name tributylamine is deprecated.) | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.002.781 |
| PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C12H27N | |
| Molar mass | 185.36 g·mol−1 |
| Appearance | Colorless to yellow liquid |
| Density | 0.78 g/cm3[1] |
| Melting point | −70 °C (−94 °F; 203 K)[1] |
| Boiling point | 214 °C (417 °F; 487 K)[1] |
| 50 mg/L (20 °C)[1] | |
| Hazards | |
| Flash point | 86 °C (187 °F; 359 K)[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tributylamine (TBA) is an organic compound with the molecular formula C12H27N. It is a colorless to yellow, hygroscopic liquid with an amine-like odor which is very poorly soluble in water.
Uses
Tributylamine has a wide range of applications. It is an intermediate in the manufacture of other chemical compounds, including quaternary ammonium compounds (such as tributylmethylammonium chloride and tributylbenzylammonium chloride), pharmaceuticals, agrochemicals, surfactants, lubricant additives, vulcanization accelerators and dyes.[2] It is also used as a catalyst (proton acceptor) and as a solvent in organic syntheses and polymerization (including polyurethanes).
References
- 1 2 3 4 5 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ Tributylamine at chemicalland21.com
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.