Transition metal alkene complex
In organometallic chemistry, a transition metal alkene complex is a coordination compound containing one or more alkene ligands. Such compounds are intermediates in many catalytic reactions that convert alkenes to other organic products.[1]
Mono- and dialkenes are often used as ligands in stable complexes.
Monoalkenes
The simplest monoalkene is ethylene. Many complexes of ethylene are known, including Zeise's salt (see figure), Rh2Cl2(C2H4)4, Cp*2Ti(C2H4), and the homoleptic Ni(C2H4)3. Substituted monoalkene include the cyclic cyclooctene, as found in chlorobis(cyclooctene)rhodium dimer. Alkenes with electron-withdrawing groups commonly bind strongly to low-valent metals. Examples of such ligands are TCNE, tetrafluoroethylene, maleic anhydride, esters of fumaric acid. These acceptors form adducts with many zero-valent metals.
Dienes and keto-alkenes
Cyclooctadiene and norbornadiene are popular chelating agents. Keto-alkenes are polyhapto ligands that stabilize highly unsaturated low valent metals as found in (Benzylideneacetone)iron tricarbonyl and tris(dibenzylideneacetone)dipalladium(0).
Trienes and polyenes
Trienes and even some tetraenes can bind to metals through several adjacent carbon centers. Common examples of such ligands are cycloheptatriene and cyclooctatetrene. The bonding is often denoted using the hapticity formalism.
- Metal alkene complexes.
2.png)
 The anion in Zeise's Salt, the first alkene complex reported in the literature.
The anion in Zeise's Salt, the first alkene complex reported in the literature.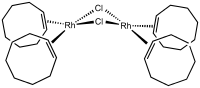
 Crabtree's catalyst, a very active catalyst for hydrogenation.
Crabtree's catalyst, a very active catalyst for hydrogenation.iron-tricarbonyl-2D-skeletal.png) (Benzylideneacetone)iron tricarbonyl.
(Benzylideneacetone)iron tricarbonyl.
Bonding
The bonding between alkenes and transition metals is described by the Dewar-Chatt-Duncanson model, which involves donation of electrons in the pi-orbital on the alkene to empty orbitals on the metal. This interaction is reinforced by back bonding that entails sharing of electrons in other metal orbitals into the otherwise empty pi-antibonding level on the alkene. Early metals of low oxidation state (Ti(II), Zr(II), Nb(III) etc.) are strong pi donors, and their alkene complexes are often described as metallacyclopropanes. Treatment of such species with acids gives the alkanes. Late metals (Ir(I), Pt(II)), which are poorer pi-donors, tend to engage the alkene as a Lewis acid-Lewis base interaction.
- Bonding images
 Orbital interactions in a metal-ethylene complex, as described by the Dewar-Chatt-Duncanson model
Orbital interactions in a metal-ethylene complex, as described by the Dewar-Chatt-Duncanson model Two extremes depictions of M---C2H4 interactions.
Two extremes depictions of M---C2H4 interactions.
Rotational barrier
The barrier for the rotation of the alkene about the M-centroid vector is a measure of the strength of the M-alkene pi-bond. Low symmetry complexes of ethylene, e.g. CpRh(C2H4)2, are suitable for analysis of the rotational barriers associated with the metal-ethylene bond. In Zeise's anion ([PtCl3(C2H4)]−) this rotational barrier cannot be assessed by NMR spectroscopy because all four protons are equivalent.
Reactions
Alkene ligands lose much of their unsaturated character upon complexation. Most famously, the alkene ligand undergoes migratory insertion, wherein it is attacked intramolecularly by alkyl and hydride ligands to form new alkyl complexes. Cationic alkene complexes are susceptible to attack by nucleophiles.
Catalysis
Metal alkene complexes are intermediates in many or most transition metal catalyzed reactions of alkenes: polymerization., hydrogenation, hydroformylation, and many other reactions.
Natural occurrence
Metal-alkene complexes are uncommon in nature, with one exception. Ethylene affects the ripening of fruit and flowers by complexation to a Cu(I) center in a transcription factor.[2]
References
- ↑ Elschenbroich, C. ”Organometallics” (2006) Wiley-VCH: Weinheim. ISBN 3-527-29390-6
- ↑ Jose M. Alonso, Anna N. Stepanova "The Ethylene Signaling Pathway" Science 2004, Vol. 306, pp. 1513-1515. doi:10.1126/science.1104812