Kjeldahl method
The Kjeldahl method or Kjeldahl digestion (Danish pronunciation: [ˈkɛldæːˀl]) in analytical chemistry is a method for the quantitative determination of organic nitrogen in chemical substances like ammonia. This method was developed by Johan Kjeldahl in 1883.[1][2]
Method
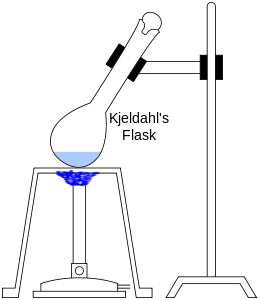
The method consists of heating a substance with sulphuric acid, which decomposes the organic substance by oxidation to liberate the reduced nitrogen as ammonium sulphate. In this step potassium sulphate is added to increase the boiling point of the medium (from 337 °C to 373 °C) . Chemical decomposition of the sample is complete when the initially very dark-coloured medium has become clear and colourless. The solution is then distilled with a small quantity of sodium hydroxide, which converts the ammonium salt to ammonia. The amount of ammonia present, and thus the amount of nitrogen present in the sample, is determined by back titration. The end of the condenser is dipped into a solution of boric acid. The ammonia reacts with the acid and the remainder of the acid is then titrated with a sodium carbonate solution by way of a methyl orange pH indicator.
- Degradation: Sample + H2SO4 → (NH4)2SO4(aq) + CO2(g) + SO2(g) + H2O(g)
- Liberation of ammonia: (NH4)2SO4(aq) + 2NaOH → Na2SO4(aq) + 2H2O(l) + 2NH3(g)
- Capture of ammonia: B(OH)3 + H2O + NH3 → NH4+ + B(OH)4−
- Back-titration: B(OH)3 + H2O + Na2CO3 → NaHCO3(aq) + NaB(OH)4(aq) + CO2(g) + H2O
In practice, this analysis is largely automated; specific catalysts accelerate the decomposition. Originally, the catalyst of choice was mercuric oxide. However, while it was very effective, health concerns resulted in it being replaced by cupric sulfate. Cupric sulfate was not as efficient as mercuric oxide, and yielded lower protein results. It was soon supplemented with titanium dioxide, which is currently the approved catalyst in all of the methods of analysis for protein in the Official Methods and Recommended Practices of AOAC International.[3]
 |  |
Applications
The Kjeldahl method's universality, precision and reproducibility have made it the internationally recognized method for estimating the protein content in foods and it is the standard method against which all other methods are judged. It is also used to assay soils, waste waters, fertilizers and other materials. It does not, however, give a measure of true protein content, as it measures nonprotein nitrogen in addition to the nitrogen in proteins. This is evidenced by the 2007 pet food incident and the 2008 Chinese milk powder scandal, when melamine, a nitrogen-rich chemical, was added to raw materials to fake high protein contents. Also, different correction factors are needed for different proteins to account for different amino acid sequences. Additional disadvantages, such as the need to use concentrated sulfuric acid at high temperature and the relatively long testing time (an hour or more), compare unfavorably with the Dumas method for measuring crude protein content.[4]
Total Kjeldahl nitrogen
Total Kjeldahl nitrogen or TKN is the sum of nitrogen in bound in organic substances, nitrogen in ammonia (NH3-N) and in ammonium (NH4+-N) in the chemical analysis of soil, water, or waste water (e.g. sewage treatment plant effluent).
Today, TKN is a required parameter for regulatory reporting at many treatment plants, and as a means of monitoring plant operations.
Conversion factors
TKN is often used as a surrogate for protein in food samples. The conversion from TKN to protein depends on the type of protein present in the sample and what fraction of the protein is composed of nitrogenous amino acids, like arginine and histidine. However, the range of conversion factors is relatively narrow. Example conversion factors, known as N factors, for foods range from 6.38 for dairy and 6.25 for meat, eggs, maize (corn) and sorghum to 5.83 for most grains; 5.95 for rice, 5.70 for wheat flour, and 5.46 for peanuts.[5]
Sensitivity
The Kjeldahl method is poorly sensitive in the original version. Other detection methods have been used to quantify NH4+ after mineralisation and distillation, achieving improved sensitivity: in-line generator of hydride coupled to a plasma atomic emission spectrometer (ICP-AES-HG, 10–25 mg/L),[6] potentiometric titration (>0.1 mg of nitrogen), zone capillary electrophoresis (1.5 µg/ml of nitrogen),[7] and ion chromatography (0.5 µg/ml).[8]
Limitations
Kjeldahl method is not applicable to compounds containing nitrogen in nitro and azo groups and nitrogen present in rings (e.g. [[pyridine],quinoline,isoquinoline]) as nitrogen of these compounds does not convert to ammonium sulfate under the conditions of this method.
See also
- Dumas method, another nitrogen analysis method
- Devarda's alloy, a powerful reducing agent for nitrate analysis
- Bicinchoninic acid assay, a colorimetric assay for protein-nitrogen
- Combustion analysis another CHN analysis method
References
- ↑ Kjeldahl, J. (1883) "Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern" (New method for the determination of nitrogen in organic substances), Zeitschrift für analytische Chemie, 22 (1) : 366-383.
- ↑ Julius B. Cohen Practical Organic Chemistry 1910 Link to online text
- ↑ AOAC International
- ↑ Dr. D. Julian McClements. "Analysis of Proteins". University of Massachusetts Amherst. Retrieved 2007-04-27.
- ↑ http://www.fao.org/docrep/006/y5022e/y5022e03.htm
- ↑ A.M.Y. Jaber, N.A. Mehanna, S.M. Sultan. Determination of ammonium and organic bound nitrogen by inductively coupled plasma emission spectroscopy. Talanta, 78 (4-5) 1298-1302, 2009.
- ↑ http://blog.pharmaphysic.fr/ecz-dosage-azote-kjeldahl/#more-592
- ↑ http://blog.pharmaphysic.fr/eviter-distillation-methode-kjeldahl/#more-83
- Wastewater Engineering: Treatment and Reuse, Metcalf & Eddy