Thorpe–Ingold effect
The Thorpe–Ingold effect, gem-dimethyl effect, or angle compression is an effect observed in organic chemistry where increasing the size of two substituents on a tetrahedral center leads to enhanced reactions between parts of the other two substituents. The effect was first reported by Beesley, Thorpe, and Ingold in 1915 as part of a study of cyclization reactions.[1]
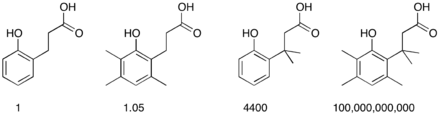
One illustration of this effect is found in the comparative rates of lactone formation (lactonization) of various 2-hydroxybenzenepropionic acids. The placement of an increasing number of methyl groups accelerates the cyclization process.[2]
A common application of this effect is addition of a quaternary carbon (e.g., a gem-dimethyl group) in an alkyl chain to increase the reaction rate and/or equilibrium constant of cyclization reactions. An example of this is an olefin metathesis reaction:[3]
One proposed explanation for this effect is that the increased size of the substituents increases the angle between them. As a result, the angle between the other two substituents decreases. By moving them closer together, reactions between them are accelerated. It is thus a kinetic effect.
The effect also has some thermodynamic contribution as the in silico strain energy decreases on going from cyclobutane to 1-methylcyclobutane and 1,1-dimethylcyclobutane by a value between 8 kcal/mole[4] and 1.5 kcal/mole.[5] A noteworthy example of Thorpe-Ingold effect in supramolecular catalysis is given by diphenylmethane derivatives provided with guanidinium groups.[6] This compounds are active in the cleavage of the RNA model compound HPNP. Substitution of the methylene group of the parent diphenylmethane spacer with cyclohexylidene and adamantylidene moieties enhances catalytic efficiency, with gem dialkyl effect accelerations of 4.5 and 9.1, respectively.
References
- ↑ Beesley, Richard Moore; Ingold, Christopher Kelk; Thorpe, Jocelyn Field (1915). "CXIX.–The formation and stability of spiro-compounds. Part I. Spiro-Compounds from cyclohexane". J. Chem. Soc., Trans. 107: 1080. doi:10.1039/CT9150701080.
- ↑ Michael N. Levine, Ronald T. Raines "Trimethyl lock: a trigger for molecular release in chemistry, biology, and pharmacology (perspective)" Chem. Sci., 2012, volume 3, 2412–2420. doi:10.1039/C2SC20536J
- ↑ Fürstner, A; Langemann, K. (1996). "A Concise Total Synthesis of Dactylol via Ring Closing Metathesis". J. Org. Chem. 61 (25): 8746–8749. doi:10.1021/jo961600c.
- ↑ Conventional Strain Energy in Dimethyl-Substituted Cyclobutane and the gem-Dimethyl Effect Ashley L. Ringer† and David H. Magers J. Org. Chem. 2007, 72, 2533–2537 doi:10.1021/jo0624647
- ↑ The gem-Dimethyl Effect Revisited Steven M. Bachrach J. Org. Chem. 2008, 73, 2466–2468 doi:10.1021/jo702665r
- ↑ Guanidine−Guanidinium Cooperation in Bifunctional Artificial Phosphodiesterases Based on Diphenylmethane Spacers; gem-Dialkyl Effect on Catalytic Efficiency Riccardo Salvio, Luigi Mandolini and Claudia Savelli J. Org. Chem. 2013, 78, 7259-7263 doi:10.1021/jo401085z