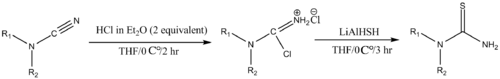Thiourea
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Thiourea | |||
| Other names
Thiocarbamide | |||
| Identifiers | |||
| 3D model (JSmol) |
|||
| 605327 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.494 | ||
| 1604 | |||
| KEGG | |||
| PubChem CID |
|||
| RTECS number | YU2800000 | ||
| UNII | |||
| UN number | 2811 | ||
| |||
| |||
| Properties | |||
| CH4N2S | |||
| Molar mass | 76.12 g/mol | ||
| Appearance | white solid | ||
| Density | 1.405 g/ml | ||
| Melting point | 182 °C (360 °F; 455 K) | ||
| 142 g/l (25 °C) | |||
| -42.4·10−6 cm3/mol | |||
| Hazards | |||
| EU classification (DSD) (outdated) |
Carc. Cat. 3 Repr. Cat. 3 Harmful (Xn) Dangerous for the environment (N) | ||
| R-phrases (outdated) | R22, R40, R51/53, R63 | ||
| S-phrases (outdated) | (S2), S36/37, S61 | ||
| NFPA 704 | |||
| Related compounds | |||
| Related compounds |
Urea | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Thiourea (/ˌθaɪəjʊˈriːə, ˌθaɪoʊ-/[1][2]) is an organosulfur compound with the formula SC(NH2)2 . It is structurally similar to urea, except that the oxygen atom is replaced by a sulfur atom, but the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis. "Thioureas" refers to a broad class of compounds with the general structure (R1R2N)(R3R4N)C=S. Thioureas are related to thioamides, e.g. RC(S)NR2, where R is methyl, ethyl, etc.

Structure and bonding
Thiourea is a planar molecule. The C=S bond distance is 1.60±0.1 Å for thiourea (as well as many of its derivatives). The material has the unusual property of changing to ammonium thiocyanate upon heating above 130 °C. Upon cooling, the ammonium salt converts back to thiourea.
Thiourea occurs in two tautomeric forms. In aqueous solutions the thione form predominates. The thiol form, which is also known as an isothiourea, can be encountered in substituted compounds such as isothiouronium salts.
Production
The global annual production of thiourea is around 10,000 tons.[3] About 40% is produced in Germany, another 40% in China, and 20% in Japan. Thiourea can be produced from ammonium thiocyanate, but more commonly it is produced by the reaction of hydrogen sulfide with calcium cyanamide in the presence of carbon dioxide.[3]
Synthesis of substituted thioureas
Many derivatives of thiourea are useful in organocatalysis. N,N′-unsubstituted thioureas can be generally prepared by treating the corresponding cyanamide with "LiAlHSH" in the presence of 1 N HCl in anhydrous diethyl ether. The "LiAlHSH" is prepared by treating lithium aluminium hydride with elemental sulfur.[4]
Alternatively, N,N′-disubstituted thioureas can be prepared by coupling two amines with thiophosgene:[5]
- R2NH + R′2NH + CSCl2 + 2 C5H5N → (R2N)(R′2N)CS + 2 C5H5NH+Cl−
Amines also condense with thiocyanates to give thioureas:[6]
- R2NH + R′NCS → (R2N)(R′(H)N)CS
Applications
The main application of thiourea is in textile processing.[3]
Organic synthesis
Thiourea reduces peroxides to the corresponding diols.[7] The intermediate of the reaction is an unstable endoperoxide which can only be identified at −100 °C. Epidioxide is similar to epoxide except with two oxygen atoms. This intermediate reduces to diol by thiourea.
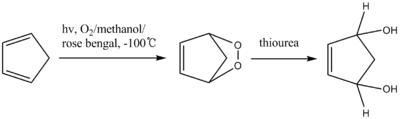
Thiourea is also used in the reductive workup of ozonolysis to give carbonyl compounds.[8] Dimethyl sulfide is also an effective reagent for this reaction, but it is highly volatile (b.p. 37 °C) and has an obnoxious odor whereas thiourea is odorless and conveniently non-volatile (reflecting its polarity).

Source of sulfide
Thiourea is commonly employed as a source of sulfide, e.g. for converting alkyl halides to thiols. Such reactions proceed via the intermediacy of isothiuronium salts. The reaction capitalizes on the high nucleophilicity of the sulfur center and easy hydrolysis of the intermediate isothiouronium salt:
- CS(NH2)2 + RX → RSC(NH
2)+
2X− - RSC(NH
2)+
2X−
+ 2 NaOH → RSNa + OC(NH2)2 + NaX - RSNa + HCl → RSH + NaCl
In this example, ethane-1,2-dithiol is prepared from 1,2-dibromoethane:[9]
- C2H4Br2 + 2 SC(NH2)2 → [C2H4(SC(NH2)2)2]Br2
- [C2H4(SC(NH2)2)2]Br2 + 2 KOH → C2H4(SH)2 + 2 OC(NH2)2 + 2 KBr
Like thioamides, thiourea can serve as a source of sulfide upon reaction with soft metal ions. For example, mercury sulfide forms when mercuric salts in aqueous solution are treated with thiourea:
- Hg2+ + SC(NH2)2 + H2O → HgS + OC(NH2)2 + 2 H+
Precursor to heterocycles
Thioureas are used a building blocks to pyrimidine derivatives. Thus thioureas condense with β-dicarbonyl compounds.[10] The amino group on the thiourea initially condenses with a carbonyl, followed by cyclization and tautomerization. Desulfurization delivers the pyrimidine.
Similarly, aminothiazoles can be synthesized by the reaction of α-haloketones and thiourea.[11]
The pharmaceuticals thiobarbituric acid and sulfathiazole are prepared using thiourea.[3] 4-Amino-3-hydrazino-5-mercapto-1,2,4-triazole is prepared by the reaction of thiourea and hydrazine.
Silver polishing
According to the label on the consumer product, the liquid silver cleaning product TarnX contains thiourea, a detergent, and sulfamic acid. A lixiviant for gold and silver leaching can be created by selectively oxidizing thiourea, bypassing the steps of cyanide use and smelting.[12]
Organocatalysis
Substituted thioureas are useful catalysts for organic synthesis. The phenomenon is called thiourea organocatalysis.[13]
Other uses
Other industrial uses of thiourea include production of flame retardant resins, and vulcanization accelerators.
Thiourea is used as an auxiliary agent in diazo paper, light-sensitive photocopy paper and almost all other types of copy paper.
It is also used to tone silver-gelatin photographic prints.
Thiourea is used in the Clifton-Phillips and Beaver bright and semi-bright electroplating processes.[14] It is also used in a solution with tin(II) chloride as an electroless tin plating solution for copper printed circuit boards.
Safety
The LD50 for thiourea is 125 mg/kg for rats (oral).[15]
A goitrogenic effect (enlargement of the thyroid gland) has been reported for chronic exposure, reflecting the ability of thiourea to interfere with iodide uptake.[3]
References
- ↑ "Thiourea". Oxford Dictionaries. Oxford University Press. Retrieved 2016-01-21.
- ↑ "Thiourea". Merriam-Webster Dictionary. Retrieved 2016-01-21.
- 1 2 3 4 5 Bernd Mertschenk, Ferdinand Beck, Wolfgang Bauer "Thiourea and Thiourea Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry 2002 by Wiley-VCH Verlag GmbH & Co. KGaA. All rights reserved. doi:10.1002/14356007.a26_803
- ↑ Koketsu, Mamoru; Kobayashi, Chikashi; Ishihara, Hideharu (2003). "Synthesis of N-aryl-S-alkylthiocarbamates". Heteroatom Chemistry. 14 (4): 374–378. doi:10.1002/hc.10163.
- ↑ Yi-Bo Huang, Wen-Bin Yi, and Chun Cai "Thiourea Based Fluorous Organocatalyst" Top Curr Chem 2012, vol. 308, p. 191–212. doi:10.1007/128_2011_248
- ↑ Miyabe, H.; Takemoto, Y. "Discovery and application of asymmetric reaction by multifunctional thioureas" Bull Chem Soc Jpn 2008, vol. 81, p785ff.
- ↑ C. Kaneko; A. Sugimoro & S. Tanaka (1974). "A facile one-step synthesis of cis-2-cyclopentene and cis-2-cyclohexene-1,4-diols from the corresponding cyclodienes". Synthesis. 1974 (12): 876–877. doi:10.1055/s-1974-23462.
- ↑ Gupta, D., Soman, G., and Dev, S. (1982). "Thiourea, a convenient reagent for the reductive cleavage of olefin ozonolysis products". Tetrahedron. 38 (20): 3013–3018. doi:10.1016/0040-4020(82)80187-7.
- ↑ Speziale, A. J. (1963). "Ethanedithiol". Org. Synth.; Coll. Vol., 4, p. 401
- ↑ Foster, H. M., and Snyder, H. R. (1963). "4-Methyl-6-hydroxypyrimidine". Org. Synth.; Coll. Vol., 4, p. 638
- ↑ Dodson, R. M. & King, L. C. (1945). "The reaction of ketones with halogens and thiourea". J. Am. Chem. Soc. 67 (12): 2242–2243. PMID 21005695. doi:10.1021/ja01228a059.
- ↑ Anthony Esposito. "Peñoles, UAM unveil pilot thiourea Au-Ag leaching plant - Mexico". Business News Americas (July 13, 2007).
- ↑ R. Schreiner, Peter (2003). "Metal-free organocatalysis through explicit hydrogen bonding interactions". Chem. Soc. Rev. 32: 289–296. PMID 14518182. doi:10.1039/b107298f.
- ↑ 81st Universal Metal Finishing Guidebook. Metal Finishing Magazine. Fall 2013. p. 285. ISSN 0026-0576.
- ↑ http://gis.dep.wv.gov/tri/cheminfo/msds1385.txt
Further reading
- Patai, S., ed. (1977). The Chemistry of double-bonded functional groups. New York, NY: John Wiley & Sons. pp. 1355–1496. ISBN 0-471-92493-8.