Fluoroboric acid
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Tetrafluoroboric acid | |
| Other names
tetrafluoroboric acid, oxonium tetrafluoroboranuide, oxonium tetrafluoridoborate(1-) | |
| Identifiers | |
| |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.037.165 |
| EC Number | 240-898-3 |
| 21702 | |
| MeSH | Fluoroboric+acid |
| PubChem CID |
|
| RTECS number | ED2685000 |
| UNII | |
| UN number | 1775 |
| |
| |
| Properties | |
| BF4H | |
| Molar mass | 87.81 g·mol−1 |
| Appearance | Colourless liquid |
| Melting point | −90 °C (−130 °F; 183 K) |
| Boiling point | 130 °C (266 °F; 403 K) |
| Acidity (pKa) | ~1.8 (MeCN solution)[1] |
| Hazards | |
| Safety data sheet | External MSDS |
| EU classification (DSD) (outdated) |
|
| R-phrases (outdated) | R34 |
| S-phrases (outdated) | (S1/2), S26, S27, S45 |
| NFPA 704 | |
| Related compounds | |
| Related compounds |
Hydrogen fluoride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
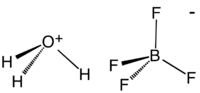
Fluoroboric acid or tetrafluoroboric acid is an inorganic compound with the chemical formula H
3OBF
4.
It is mainly produced as a precursor to other fluoroborate salts.[2] It is a strong acid. Fluoroboric acid is corrosive and attacks the skin. It is available commercially as a solution in water and other solvents such as diethyl ether. It is a strong acid with a weakly coordinating, non-oxidizing conjugate base.[1] It is structurally similar to perchloric acid, but lacks the hazards associated with oxidants.
Structure and production
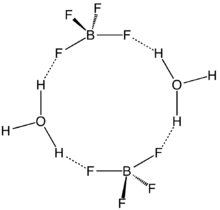
Although the solvent-free HBF4 has not been isolated, its solvates are well characterized. These salts consist of protonated solvent as a cation, e.g., H3O+ and H
5O+
2, and the tetrahedral BF−
4 anion. The anion and cations are strongly hydrogen-bonded.[3]
Aqueous solutions of HBF4 are produced by dissolving boric acid in aqueous hydrofluoric acid.[4][5] Three equivalents of HF react to give the intermediate boron trifluoride and the fourth gives fluoroboric acid:
- B(OH)3 + 4 HF → H3O+ + BF−
4 + 2 H2O
- B(OH)3 + 4 HF → H3O+ + BF−
Anhydrous solutions can be prepared by treatment of aqueous fluoroboric acid with acetic anhydride.[6]
Acidity
The acidity of fluoroboric acid is complicated by the fact that the name refers to several different species H(OEt2)+BF−
4, H3O+BF−
4, and HF.BF3 – each with a different acidity. The aqueous pKa is quoted as −0.44.[2] Titration of NBu+
4BF−
4 in acetonitrile solution indicates that HBF4, i.e., HF.BF3, has a pKa of 1.6 in that solvent. Its acidity is thus comparable to that of fluorosulfonic acid.[1]
Applications
Fluoroboric acid is the principal precursor to fluoroborate salts, which are typically prepared by treating the metal oxides with fluoroboric acid. The inorganic salts are intermediates in the manufacture of flame-retardant materials and glazing frits, and in electrolytic generation of boron. HBF4 is also used in aluminum etching and acid pickling.
Organic chemistry
HBF4 is used as a catalyst for alkylations and polymerizations. In carbohydrate protection reactions, ethereal fluoroboric acid is an efficient and cost-effective catalyst for transacetalation and isopropylidenation reactions. Acetonitrile solutions cleave acetals and some ethers. Many reactive cations have been obtained using fluoroboric acid, e.g. tropylium tetrafluoroborate (C
7H+
7BF−
4), triphenylmethyl tetrafluoroborate (Ph
3C+
BF−
4), triethyloxonium tetrafluoroborate (Et
3O+
BF−
4), and benzenediazonium tetrafluoroborate (PHN+
2BF−
4).
Galvanic cells
Aqueous HBF4 is used as an electrolyte in galvanic cell oxygen sensor systems, which consist of an anode, cathode, and oxygen-permeable membrane. The solution of HBF4 is able to dissolve lead(II) oxide from the anode in the form of lead tetrafluoroborate while leaving the rest of the system unchanged.
Metal plating
A mixture of CrO3, HBF4, and sulfonic acids in conjunction with a cathode treatment give tin-plated steel. Tin(I) fluoroborate/fluoroboric acid mixtures and organic reagents are used as the electrolyte in the cathode treatment of the tin plating process. Similar processes of electrodeposition and electrolytic stripping are used to obtain specific metal alloys.
A 10% solution of fluoroboric acid, tin fluoroborate and thiourea is used to immersion tin plate the copper traces of printed circuit boards for protection and easier soldering. This is not to be confused with electroless plating of the traces as the solution, commercially sold as Liquid Tin, does not contain the chemicals required to reduce tin (II) tetrafluoroborate to metallic tin. Rather, this chemical relies on the low pH created by the fluoroboric acid to increase the reduction potential of tin vs copper to the point to where tin preferentially plates on the copper traces.
Other fluoroboric acids
A series of fluoroboric acids is known in aqueous solutions. The series can be presented as follows:[7]
- H[B(OH)4]
- H[BF(OH)3]
- H[BF2(OH)2]
- H[BF3(OH)]
- H[BF4]
See also
References
- 1 2 3 Kütt, A., et al., "Equilibrium Acidities of Superacids", J. Org. Chem. 2010, volume 76, pp. 391-395. doi:10.1021/jo101409p
- 1 2 Gregory K. Friestad, Bruce P. Branchaud "Tetrafluoroboric Acid" E-Eros Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt035
- ↑ Mootz, D.; Steffen, M. "Crystal structures of acid hydrates and oxonium salts. XX. Oxonium tetrafluoroborates H3OBF4, [H5O2]BF4, and [H(CH3OH)2]BF4", Zeitschrift für Anorganische und Allgemeine Chemie 1981, vol. 482, pp. 193-200. doi:10.1002/zaac.19814821124
- ↑ Brotherton, R. J.; Weber, C. J.; Guibert, C. R.; Little, J. L. (2005), "Boron Compounds", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a04_309
- ↑ Flood, D. T. (1933). "Fluorobenzene" (PDF). Org. Synth. 13: 46.; Coll. Vol., 2, p. 295
- ↑ Wudl, F.; Kaplan, M. L., "2,2′-Bi-L,3-Dithiolylidene (Tetrathiafulvalene, TTF) and its Radical Cation Salts" Inorg. Synth. 1979, vol. 19, 27. doi:10.1002/9780470132500.ch7
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
Further reading
- Albert, R.; Dax, K.; Pleschko, R.; Stütz, A. E. (1985). "Tetrafluoroboric acid, an efficient catalyst in carbohydrate protection and deprotection reactions". Carbohydrate Research. 137: 282–290. doi:10.1016/0008-6215(85)85171-5.
- Bandgar, B. P.; Patil, A. V.; Chavan, O. S. (2006). "Silica supported fluoroboric acid as a novel, efficient and reusable catalyst for the synthesis of 1,5-benzodiazepines under solvent-free conditions". Journal of Molecular Catalysis A: Chemical. 256 (1–2): 99–105. doi:10.1016/j.molcata.2006.04.024.
- Heintz, R. A.; Smith, J. A.; Szalay, P. S.; Weisgerber, A.; Dunbar, K. R. (2002). "Homoleptic Transition Metal Acetonitrile Cations with Tetrafluoroborate or Trifluoromethanesulfonate Anions". Inorganic Syntheses. 33: 75–83. doi:10.1002/0471224502.
- Housecroft, C. E.; Sharpe, A. G. (2004). Inorganic Chemistry (2nd ed.). Prentice Hall. p. 307. ISBN 978-0130399137.
- Meller, A. (1988). "Boron". Gmelin Handbook of Inorganic Chemistry. 3. New York: Springer-Verlag. pp. 301–310.
- Perry, D. L.; Phillips, S. L. (1995). Handbook of Inorganic Compounds (1st ed.). Boca Raton: CRC Press. p. 1203. ISBN 9780849386718.
- Wamser, C. A. (1948). "Hydrolysis of Fluoboric Acid in Aqueous Solution". Journal of the American Chemical Society. 70 (3): 1209–1215. doi:10.1021/ja01183a101.
- Wilke-Dörfurt, E.; Balz, G. (1927). "Zur Kenntnis der Borfluorwasserstoffsäure und ihrer Salze". Zeitschrift für Anorganische und Allgemeine Chemie. 159 (1): 197–225. doi:10.1002/zaac.19271590118.
External links
- "Fluoroboric Acid ICSC: 1040". INCHEM.
