Clostridium tetani
| Clostridium tetani | |
|---|---|
 | |
| Clostridium tetani with characteristic 'tennis racket' appearance. | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Firmicutes |
| Class: | Clostridia |
| Order: | Clostridiales |
| Family: | Clostridiaceae |
| Genus: | Clostridium |
| Species: | C. tetani |
| Binomial name | |
| Clostridium tetani Flügge, 1881 | |
Clostridium tetani is a rod-shaped, anaerobic species of pathogenic bacteria, of the genus Clostridium. Like other Clostridium genus species, it is Gram-positive, and its appearance on a gram stain resembles tennis rackets or drumsticks.[1] C. tetani is found as spores in soil or in the gastrointestinal tract of animals. C. tetani produces a potent biological toxin, tetanospasmin, and is the causative agent of tetanus, a disease characterized by painful muscular spasms that can lead to respiratory failure and, in up to 10% of cases, death.
History
Tetanus was known to ancient people, who recognized the relationship between wounds and fatal muscle spasms. In 1884, Arthur Nicolaier isolated the strychnine-like toxin of tetanus from free-living, anaerobic soil bacteria. The etiology of the disease was further elucidated in 1890 by Antonio Carle and Giorgio Rattone, who demonstrated the transmissibility of tetanus for the first time. They produced tetanus in rabbits by injecting their sciatic nerve with pus from a fatal human tetanus case in that same year. In 1889, C. tetani was isolated from a human victim, by Kitasato Shibasaburō, who later showed that the organism could produce disease when injected into animals, and that the toxin could be neutralized by specific antibodies. In 1897, Edmond Nocard showed that tetanus antitoxin induced passive immunity in humans, and could be used for prophylaxis and treatment. Tetanus toxoid vaccine was developed by P. Descombey in 1924, and was widely used to prevent tetanus induced by battle wounds during World War II.[2]
Characteristics
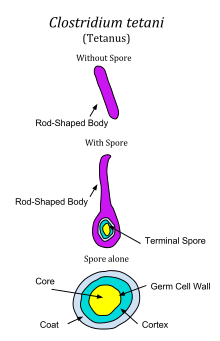
C. tetani is a rod-shaped, obligate anaerobe which stains Gram positive in fresh cultures; established cultures may stain Gram negative.[1] During vegetative growth, the organism cannot survive in the presence of oxygen, is heat-sensitive and exhibits flagellar motility. As the bacterium matures, it develops a terminal spore, which gives the organism its characteristic appearance. C. tetani spores are extremely hardy as they are resistant to heat and most antiseptics.[3] The spores are distributed widely in manure-treated soils and can also be found on human skin. [2]The incubation period is typically 3-21 days. The period of illness is a bit shorter and that is 14 days. Lastly C. tetani is not contagious. [2].
Vaccination
Tetanus can be prevented through the highly effective tetanus vaccine, which is a tetanus toxin inactivated with formaldehyde to be immunogenic but not pathogenic. The vaccine can be formulated as simple or adsorbed tetanus vaccine, combined tetanus and killed polio vaccine, or the older (diphtheria, tetanus, pertussis) (DPT) vaccine. Side effects are rare, but if they do occur, include fever, pain (sometimes long lasting) at the injection site, unexplained crying in infants, and irritability in older children or adults. Severe reactions are extremely rare and include anaphylaxis, seizures and encephalopathy. All infants are recommended to receive the vaccine at 2, 4, 6, and 15 months of age ({[4]}). A fifth booster dose should be given at 4–6 years of age ({[4]}). After that, it should be given every 10 years. However, if a bite, scratch, or puncture occurs more than five years after the last dose of vaccine, the patients should receive another dose of vaccine.
Toxicity
C. tetani usually enters a host through a wound to the skin, then it replicates. High risk individuals are people exposed to soil or animal feces. The spores are widely distributed in soil and in the intestines and feces of horses, sheep, cattle, dogs, cats, rats, guinea pigs, and chickens. Manure-treated soil may contain large numbers of spores. In agricultural areas, a significant number of human adults may harbor the organism. The spores can also be found on skin surfaces and in contaminated heroin.[2] Once an infection is established, C. tetani produces two exotoxins, tetanolysin and tetanospasmin. Eleven strains of C. tetani have been identified, which differ primarily in flagellar antigens and in their ability to produce tetanospasmin. The genes for toxin production are encoded on a plasmid which is present in all toxigenic strains, and all strains that are capable of producing toxin produce identical toxins.[5]
Tetanolysin serves no known benefit to C. tetani. Tetanospasmin is a neurotoxin that causes the clinical manifestations of tetanus. Tetanus toxin is generated in living bacteria, and is released when the bacteria lyse, such as during spore germination or vegetative growth. A minimal amount of spore germination and vegetative cell growth are required for toxin production.[5]
Tetanus toxin is a potent neurotoxin. On the basis of weight, tetanospasmin is one of the most potent toxins known (based on tests conducted on mice). The estimated minimum human lethal dose is 2.5 nanograms per kilogram of body weight, or 175 nanograms in a 70 kg (154 lb) human.[2] The only toxins more lethal to mice are botulinum toxin, produced by close relative Clostridium botulinum and the exotoxin produced by Corynebacterium diphtheriae, the causative agent of diphtheria. It should be noted, however, that humans and other animals may react to specific toxins differently from mice, and that the overall lethality of a specific toxin likely varies between different animals.
Tetanospasmin is a zinc-dependent metalloproteinase that is structurally similar to botulinum toxin, but with different effects. C. tetani synthesizes tetanospasmin as a single 150kDa polypeptide progenitor toxin that is then cleaved by a protease into two fragments; fragment A (a 50kDa "light chain") and fragment B (a 100 kDa "heavy chain") which remain connected via a disulfide bridge. Cleavage of the progenitor toxin into A and B fragments can be induced artificially by trypsin.[5]
Toxin action
Tetanospasmin released in the wound is absorbed into the circulation and reaches the ends of motor neurons all over the body. The toxin acts at several sites within the central nervous system, including nerve terminals, the spinal cord, and brain, and within the sympathetic nervous system. By binding to peripheral motor neuron terminals, the toxin enters the nerve axons, and is transported across synaptic junctions to the nerve-cell body in the brain stem and spinal cord by retrograde intraneuronal transport, until it reaches the central nervous system, where it rapidly binds to gangliosides at the presynaptic membrane of inhibitory motor nerve endings.[2]
The clinical manifestations of tetanus are caused when tetanus toxin blocks inhibitory impulses, by interfering with the release of neurotransmitters, including glycine and gamma-aminobutyric acid. These inhibitory neurotransmitters inhibit the alpha motor neurons. With diminished inhibition, the resting firing rate of the alpha motor neuron increases, producing rigidity, unopposed muscle contraction and spasm. Characteristic features are risus sardonicus (a rigid smile), trismus (commonly known as "lock-jaw"), and opisthotonus (rigid, arched back). Seizures may occur, and the autonomic nervous system may also be affected. Tetanospasmin appears to prevent the release of neurotransmitters by selectively cleaving a component of synaptic vesicles called synaptobrevin II.[5] Loss of inhibition also affects preganglionic sympathetic neurons in the lateral gray matter of the spinal cord and produces sympathetic hyperactivity and high circulating catecholamine levels. Hypertension and tachycardia alternating with hypotension and bradycardia may develop.[6][7]
Genome structure
Clostridium tetani has a genome that contains 2.80 Mbp with 2,373 protein coding genes.[8]
References
- 1 2 Ryan, KJ; Ray, CG, eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. ISBN 0-8385-8529-9.
- 1 2 3 4 5 6 Centers for Disease Control and Prevention (2006). "Tetanus" (PDF). In Atkinson, W; Hamborsky, J; McIntyre, L; et al. Epidemiology and Prevention of Vaccine-Preventable Diseases (The Pink Book) (10th ed.). Public Health Foundation.
- ↑ Madigan, M; Martinko, J, eds. (2005). Brock Biology of Microorganisms (11th ed.). Prentice Hall. ISBN 0-13-144329-1.
- 1 2 http://www.cdc.gov/vaccines/parents/downloads/parent-ver-sch-0-6yrs.pdf
- 1 2 3 4 Todar, Ken (2005) Pathogenic Clostridia, Ken Todar's Microbial World, University of Wisconsin - Madison.
- ↑ Loscalzo, Joseph; Fauci, Anthony S.; Braunwald, Eugene; Dennis L. Kasper; Hauser, Stephen L; Longo, Dan L. (2008). Harrison's principles of internal medicine. McGraw-Hill Medical. ISBN 0-07-146633-9.
- ↑ "Tetanus in Emergency Medicine". Emedicine. Retrieved 2011-09-01.
- ↑ Bruggemann, H.; Baumer, S.; Fricke, WF.; Wiezer, A.; Liesegang, H.; Decker, I.; Herzberg, C.; Martinez-Arias, R.; et al. (Feb 2003). "The genome sequence of Clostridium tetani, the causative agent of tetanus disease". Proc Natl Acad Sci U S A. 100 (3): 1316–21. PMC 298770
 . PMID 12552129. doi:10.1073/pnas.0335853100.
. PMID 12552129. doi:10.1073/pnas.0335853100.
Further reading
- Clinical Micropebnishy. ISBN 0-940780-49-6.
External links
- Pathema-Clostridium Resource
- Centers for Disease Control and Prevention (2012). "Ch. 20: Tetanus". In Atkinson, W; Wolfe, S; Hamborsky, J. Epidemiology and Prevention of Vaccine-Preventable Diseases (12th ed.). Washington DC: Public Health Foundation. pp. 291–300.