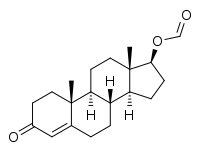
Testosterone formate
 | |
| Clinical data | |
|---|---|
| Synonyms | Testosterone carboxylate; Testosterone methanoate |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C20H28O3 |
| Molar mass | 316.441 g/mol |
| 3D model (JSmol) | |
| |
| |
Testosterone formate, also known as testosterone carboxylate or testosterone methanoate, as well as androst-4-en-17β-ol-3-one 17β-formate, is a synthetic, steroidal androgen and an androgen ester – specifically, the C17β formate ester of testosterone – which was first synthesized in the 1930s and was never marketed.[1][2][3][4]
See also
References
- ↑ Samuel H. Yalkowsky; Yan He; Parijat Jain (19 April 2016). Handbook of Aqueous Solubility Data, Second Edition. CRC Press. pp. 1233–. ISBN 978-1-4398-0246-5.
- ↑ R T Blickenstaff (2 December 2012). Antitumor Steroids. Academic Press. pp. 76–. ISBN 978-0-323-13916-8.
- ↑ Edith Josephy; F. Radt (1 December 2013). Elsevier’s Encyclopaedia of Organic Chemistry: Series III: Carboisocyclic Condensed Compounds. Springer. pp. 3019–. ISBN 978-3-662-25863-7.
- ↑ Koch, F. C. (1937). "RECENT ADVANCES IN THE FIELD OF ANDROGENS". Cold Spring Harbor Symposia on Quantitative Biology. 5 (0): 34–43. ISSN 0091-7451. doi:10.1101/SQB.1937.005.01.004.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.