Butyl group
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula −C4H9, derived from either of the two isomers of butane.
The isomer n-butane can connect in two ways, giving rise to two "-butyl" groups:
- If it connects at one of the two terminal carbon atoms, it is normal butyl or n-butyl: CH3−CH2−CH2−CH2− (fully systematic name: butyl)
- If it connects at one of the non-terminal (internal) carbon atoms, it is secondary butyl or sec-butyl: CH3−CH2−CH(CH3)− (fully systematic name: 1-methylpropyl)
The second, branched isomer of butane, isobutane, can also connect in two ways, giving rise to two additional groups:
- If it connects at one of the three terminal carbons, it is isobutyl: (CH3)2CH−CH2− (fully systematic name: 2-methylpropyl)
- If it connects at the central carbon, it is tertiary butyl, tert-butyl or t-butyl: (CH3)3C− (fully systematic name: 1,1-dimethylethyl)
Nomenclature
According to IUPAC nomenclature, "isobutyl", "sec-butyl", and "tert-butyl" are all retained names. In the convention of skeletal formulas, every line ending and line intersection specifies a carbon atom (unless otherwise indicated) saturated with single-linked hydrogen atoms (unless otherwise indicated). The "R" symbol indicates any radical or other non-specific functional group.
| Skeletal formula | Common name | IUPAC name | Systematic name | Alternate notation |
|---|---|---|---|---|
| n-butyl | butyl | butyl | butan-1-yl | |
 |
sec-butyl | sec-butyl | 1-methylpropyl | butan-2-yl |
| isobutyl | isobutyl | 2-methylpropyl | 2-methylpropan-1-yl | |
 |
tert-butyl | tert-butyl | 1,1-dimethylethyl | 2-methylpropan-2-yl |
Butyl is the largest substituent for which trivial names are commonly used for all isomers.
The butyl group's carbon that is connected to the rest (R) of the molecule is called the RI or R-prime carbon . The prefixes sec (from "secondary") and tert (from "tertiary") refer to the number of additional side chains (or carbons) connected to the first butyl carbon. The prefix "iso" (from "isomer") means "equal" while the prefix 'n-' stands for "normal".
Example
The four isomers of "butyl acetate" demonstrate these four isomeric configurations. Here, the acetate radical appears in each of the positions where the "R" symbol is used in the chart above:
 |
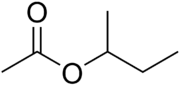 |
 |
 |
Etymology
Alkyl radicals are often considered as a series, a progression sequenced by the number of carbon atoms involved. In that progression, Butyl (containing 4 carbon atoms) is the fourth, and the last to be named for its history. The word "butyl" is derived from butyric acid, a four-carbon carboxylic acid found in rancid butter.[1] The name "butyric acid" comes from Latin butyrum, butter. Subsequent alkyl radicals in the series are simply named from the Greek number that indicates the number of carbon atoms in the group: pentyl, hexyl, heptyl, etc.
tert-Butyl effect
The tert-butyl substituent is very bulky and is used in chemistry for kinetic stabilization, as are other bulky groups such as the related trimethylsilyl group. The effect of the tert-butyl group on the progress of a chemical reaction is called the tert-butyl effect, illustrated in the Diels-Alder reaction below. Compared to a hydrogen substituent, the tert-butyl substituent accelerates the reaction rate by a factor of 240.[2]

References
- ↑ Harper, Douglas. "butane. Dictionary.com". Retrieved 9 Mar 2016.
- ↑ Factors affecting ease of ring formation. The effect of anchoring substitution on the rate of an intramolecular diels-alder reaction with furan-diene Serge Cauwberghs and Pierre J. De Clercq B. Tinant and J. P. Declercq Tetrahedron Letters Volume 29, Issue 20 , 1988, Pages 2493-2496 doi:10.1016/S0040-4039(00)87916-2