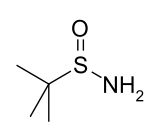
''tert''-Butanesulfinamide
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-Methyl-2-propanesulfinamide | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.108.188 |
| PubChem CID |
|
| |
| |
| Properties | |
| (CH3)3CS(O)NH2 | |
| Molar mass | 121.20 g/mol |
| Appearance | white to off-white crystalline solid |
| Melting point | 102 to 105 °C (216 to 221 °F; 375 to 378 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
tert-Butanesulfinamide is an organosulfur compound and a member of the class of sulfinamides. Both enantiomeric forms are commercially available and are relevant to the asymmetric synthesis of amines as a chiral ammonia equivalent.[1][2][3] This methodology was introduced in 1997 by Jonathan A. Ellman.[4]
Synthesis
Chiral tert-butanesulfinamide can be prepared by enantioselective oxidation of inexpensive di-tert-butyl disulfide to the thiosulfinate followed by disulfide bond cleavage by lithium amide. In the original scope the chiral ligand used together with vanadyl acetylacetonate was prepared by condensing a chiral aminoindanol with 3,5-di-tert-butyl salicylaldehyde.
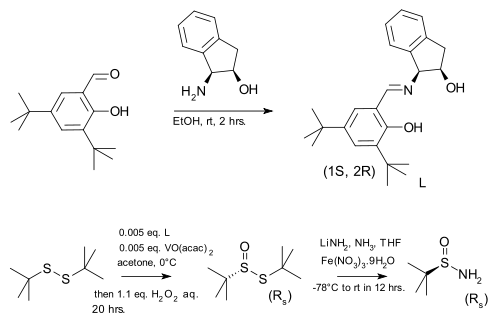 |
| tert-Butanesulfinamide synthesis |
|---|
Amine synthesis
Condensation with ketones and aldehydes yield the corresponding N-tert-butanesulfinyl aldimines and ketimines. These intermediates are more resistant to hydrolysis than other imines but more reactive towards nucleophiles. A nucleophile adds diastereoselectively over the imine group in an electrophilic addition with the tert-butanesulfinyl group acting as a chiral auxiliary. This tert-butanesulfinyl group is also a protecting group. On addition of hydrochloric acid the tert-butanesulfinyl group is removed forming the chiral primary ammonium salt or amine (from aldehyde precursor) or the chiral secondary amine (ketone precursor).
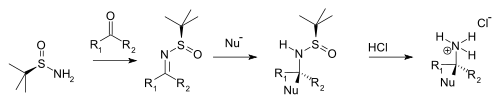 |
| tert-Butanesulfinamide chiral amine synthesis |
|---|
Typical nucleophiles are Grignard reagents, organozinc compounds, organolithium compounds, and enolates.
Chiral sulfinimines as intermediates for the asymmetric synthesis of amines have also been developed by Franklin A. Davis.[5]
Applications
tert-Butanesulfinamide has been used as an auxiliary in an asymmetric synthesis of cetirizine (more potent than the racemic mixture of the drug) starting from p-chlorobenzaldehyde and phenylmagnesium bromide:[6]
 |
| Asymmetric cetirizine synthesis |
|---|
References
- ↑ Ellman, J. A. (2003). "Applications of tert-butanesulfinamide in the asymmetric synthesis of amines". Pure and Applied Chemistry. 75: 39. doi:10.1351/pac200375010039.
- ↑ Robak, Maryann T.; Herbage, Melissa A.; Ellman, Jonathan A. (2010). "Synthesis and Applications oftert-Butanesulfinamide". Chemical Reviews. 110 (6): 100426091145060. PMID 20420386. doi:10.1021/cr900382t.
- ↑ Organic Syntheses, Vol. 82, p.157 (2005). Link
- ↑ Liu, Guangcheng; Cogan, Derek A.; Ellman, Jonathan A. (1997). "Catalytic Asymmetric Synthesis oftert-Butanesulfinamide. Application to the Asymmetric Synthesis of Amines". Journal of the American Chemical Society. 119 (41): 9913. doi:10.1021/ja972012z.
- ↑ Davis, Franklin A.; Reddy, Rajarathnam E.; Szewczyk, Joanna M.; Reddy, G. Venkat; Portonovo, Padma S.; Zhang, Huiming; Fanelli, Dean; Zhou, Ping; et al. (1997). "Asymmetric Synthesis and Properties of Sulfinimines (ThiooximeS-Oxides)". The Journal of Organic Chemistry. 62 (8): 2555–2563. PMID 11671597. doi:10.1021/jo970077e.
- ↑ Pflum, D; Krishnamurthy, D; Han, Z; Wald, S; Senanayake, C (2002). "Asymmetric synthesis of cetirizine dihydrochloride". Tetrahedron Letters. 43 (6): 923. doi:10.1016/S0040-4039(01)02294-8.