Tecemotide
Tecemotide (INN; emepepimut-S (USAN); formerly known as BLP25 or EMD 531444) is a synthetic lipopeptide that is used as antigen in an investigational therapeutic cancer vaccine (formerly known as Stimuvax, L-BLP25, BLP25 liposomal vaccine or BLP25 liposome vaccine). The investigational therapeutic cancer vaccine is designed to induce a cellular immune response to cancer cells that express MUC1, a glycoprotein antigen that is widely over-expressed on common cancers such as lung cancer, breast cancer, prostate cancer and colorectal cancer. The cellular immune response may lead then to a rejection of tumor tissue expressing the MUC1 antigen.[1]
Collaboration
Tecemotide was developed – until Clinical trial phase II – by the Canadian biotech company Biomira Inc., which changed in 2007 the company name to Oncothyreon Inc.[2] Oncothyreon is now located in Seattle, Washington, USA.
In 2001, Merck KGaA, Darmstadt, Germany, entered into a collaboration and supply agreement with Oncothyreon. In 2007, Merck KGaA acquired the exclusive worldwide marketing rights from Oncothyreon and Merck KGaA is since then entirely responsible for the further clinical development of Tecemotide.[3] In 2008, Merck KGaA acquired the manufacturing rights for Tecemotide from Oncothyreon.[4] In 2011, Ono Pharmaceutical Co., Ltd., Japan, acquired a co-development and co-marketing license for Tecemotide in Japan and Merck KGaA received an upfront payment of 5 million Euros.[5]
Structure
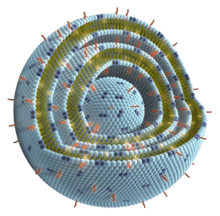
The antigen: Tecemotide
- Nonproprietary/generic names:
- International Nonproprietary Name (INN): Tecemotide
- United States Adopted Name (USAN): Emepepimut-S
- Formerly known as:
- BLP25 (Biomira lipopeptide 25) or EMD 531444
- Function:
- Tecemotide is the antigen of the cancer vaccine
- Tecemotide is a synthetic lipopeptide 27 amino acids long. The first 25 amino acids of tecemotide are derived from the mucin 1 (MUC1) sequence:
- human mucin-1 (carcinoma-associated mucin, episialin, CD227)-(107-131)-peptide (sequence 40 times repeated) fusion protein with 6-N-hexadecanoyl-L-lysylglycine
- Molecular formula:
- C124H203N33O38
- CAS Registry Number:
- 221214-84-2
- Amino acid sequence (27 amino acids):
- STAPPAHGVTSAPDTRPAPGSTAPPKG
- aa 1-25: derived from the mucin 1 (MUC1) sequence
- aa 26: the modified amino acid K is palmityl-lysin (N6-(1-oxohexadecyl)-L-lysyin)[7]
The adjuvant: MPL
3-O-Deacyl-4’-Monophosphoryl lipid A (MPL) is the adjuvant in the cancer vaccine.[8] MPL is a derivative of the lipid A molecule found in the membrane of Gram-negative bacteria. MPL is also used as adjuvant in other vaccines, e.g. Cervarix which is a vaccine against certain types of cancer-causing human papillomavirus (HPV).
Function:
- Non-specific immune stimulus
- Stimulates macrophage activation
- Stimulates antigen-presenting cells (APCs) through the toll-like receptor 4 (TLR-4)
The carrier: Lipids
Lipids:
- Cholesterol, dimyristoyl phosphatidylglycerol (DMPG) and dipalmitoyl phosphatidylcholine (DPPC)[9]
Function:
- Structure of the liposome
- Enhances uptake by APCs
The cancer vaccine: A liposomal formulation
The antigen tecemotide is anchored — together with the adjuvant MPL — in the membrane of the liposome. This liposomal formulation is the investigational therapeutic cancer vaccine (formerly known as Stimuvax, L-BLP25, BLP25 liposomal vaccine or BLP25 liposome vaccine). The cancer vaccine is a lyophilized powder, which is formulated to contain 300 μg of tecemotide and 150 μg of MPL per vial.[10]
Clinical trials
Overview and results of all trials
Tecemotide clinical trials (as of September 2, 2014)[11] sorted by (Estimated) Primary Completion Date:[12]
| Clinical trials:
Lung cancer, Breast cancer, Prostate cancer, Colorectal cancer and Multiple myeloma | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| NCT Number / Title | Other IDs | Start Date | (Estimated) Primary Completion Date | Recruitment | Conditions | Interventions | Phases | Enrollment (Patients) | Sponsor / Collaborators |
| NCT00157209 Phase IIb Randomized Controlled Study of BLP25 Liposome Vaccine for Immunotherapy of Non-Small Cell Lung Cancer | B25-LG-304 / EMR 63325-005 | August 2000 | March 2006 | Completed | Lung Neoplasms, Carcinoma, Non-Small-Cell Lung | Biological: BLP25 Liposome Vaccine plus best supportive care; Other: Best Supportive Care (BSC) | Phase 2 | 171 | Merck KGaA |
| Results: A Phase IIb clinical trial of 171 patients with inoperable stage IIIb non-small-cell lung cancer (NSCLC), in which tecemotide showed a trend towards extending median overall survival from 13.3 months for patients receiving best supportive care (BSC) to 30.6 months for patients receiving tecemotide plus BSC. Although this represents a subgroup analysis which is not statistically significant (with a nonsignificant P-value: p = 0.16), the magnitude of the difference and its durability over a prolonged period of follow up suggest an efficacy signal for the vaccine and would support further testing of tecemotide in a definitive phase III trial. Reported side effects included mild-to-moderate flu-like symptoms, gastrointestinal disturbances, and mild injection-site reactions.[13][14] | |||||||||
| NCT00157196 Safety Study of BLP25 Liposome Vaccine in Non-Small Cell Lung Cancer Patients With Unresectable Stage III Disease | B25-LG-305 / EMR 63325-006 | April 2005 | September 2009 | Completed | Carcinoma, Non-Small-Cell Lung, Lung Neoplasms | Biological: BLP25 Liposome Vaccine | Phase 2 | 22 | Merck KGaA |
| NCT00925548 STRIDE - STimulating Immune Response In aDvanced brEast Cancer | STRIDE, EMR 200038-010, 2008-005544-17 | September 2009 | August 2010 | Terminated | Breast Cancer | Biological: Tecemotide (L-BLP25) and Hormonal Treatment, Biological: Placebo of tecemotide (L-BLP25) and Hormonal Treatment, Drug: cyclophosphamide, Drug: sodium chloride (NaCl) | Phase 3 | 16 | EMD Serono |
| A thirty-site phase III trial (STRIDE) began in September 2009 on 900 women. The purpose of this study is to determine whether the addition of tecemotide to hormonal treatment is effective in prolonging progression-free survival in postmenopausal women with endocrine-sensitive inoperable locally advanced, recurrent or metastatic breast cancer.[15][16]
Results: The U.S. Food and Drug Administration (FDA) put the Phase III STRIDE trial on hold in March 2010 after a patient participating in a Phase II clinical trial with tecemotide in patients with multiple myeloma developed encephalitis.[17] The study was terminated in August 2010 (16 patients were enrolled in the study). | |||||||||
| NCT01094548 Study of Stimuvax in Patients With Slowly Progressive Multiple Myeloma With no Symptoms and Who Have Had no Chemotherapy | EMR63325-008 | February 2008 | February 2011 | Completed | Multiple Myeloma | Biological: L-BLP25, cyclophosphamide prior to first vaccination, Biological: L-BLP25 | Phase 2 | 34 | Merck KGaA |
| NCT00409188 Cancer Vaccine Study for Unresectable Stage III Non-small Cell Lung Cancer | START, EMR 63325-001 | January 2007 | August 2012 | Completed | Non-small Cell Lung Cancer | Biological: Tecemotide (L-BLP25), Biological: Placebo | Phase 3 | 1513 | EMD Serono, Merck KGaA |
| Results: The primary endpoint of overall survival was not met in the START trial.[18] However, the exploratory subgroup analysis in the START trial generated a reasonable hypothesis to warrant additional study.[19] | |||||||||
| NCT01731587 Anti-cancer MUC1-specific Immunotherapy for Unresectable Stage III Non-small Cell Lung Cancer | FINGERPRINT, EMR 63325-019, 2012-001435-31 | Withdrawn | Non-small Cell Lung Cancer (NSCLC) Stage III | Other: Biological: MUC1 peptide specific immunotherapy, Drug: Cyclophosphamide (CPA) | Phase 1 | 0 | Merck KGaA | ||
| NCT00960115 Study of EMD531444 in Subjects With Stage III Unresectable Non-small Cell Lung Cancer (NSCLC) Following Primary Chemoradiotherapy | EMR063325-009 | December 2008 | May 2014 | Completed | Non-small Cell Lung Cancer | Biological: cyclophosphamide + EMD531444 + BSC, Biological: Saline + Placebo + BSC | Phase 1, Phase 2 | 205 | Merck KGaA, Merck Serono Co., Ltd., Japan |
| Results: The analysis of EMR 63325-009, a randomized, double-blind, placebo-controlled Phase I/II study in Japanese patients with Stage III unresectable, locally advanced NSCLC who had received concurrent or sequential chemoradiotherapy (CRT), with a minimum of two cycles of platinum-based chemotherapy and radiation dose ≥50 Gy shows following results: Of the patients included in the Phase II part of the study, the majority had received concurrent CRT. The results indicate that no effect has been observed for either the primary endpoint, overall survival (OS), or for any of the secondary endpoints (progression-free survival [PFS], time to progression [TTP] and time to treatment failure). An analysis of the reported adverse events has not identified a clinically meaningful difference in the frequency between treatment groups. Although the trial was not powered to demonstrate a statistically significant difference in benefit between the two arms, Merck Serono made the recommendation to stop the investigational treatment for patients in the EMR 63325-009 study in Japan.[20] | |||||||||
| EudraCT: 2011-004822-85 A prospective, open, randomized, phase-II study of a therapeutic cancer vaccine (L-BLP25, Stimuvax) in the pre-operative treatment of women with primary breast cancer | ABCSG-34 / EMR 63325-603 | Jan 2012 | May 2014 | Active, not recruiting | Breast Cancer | L-BLP25 (Stimuvax), Cyclophosphamide (CPA), LETROZOLE, EPIRUBICIN, DOCETAXEL | Phase 2 | 400 | ABCSG (Austrian Breast & Colorectal Cancer Study Group) |
| Results:
8 May 2012[21]
26 Sept 2014[22]
| |||||||||
| NCT01507103 Tecemotide (L-BLP25) in Rectal Cancer | SPRINT, EMR063325-013, 2011-000847-25 | February 2012 | June 2014 | Active, not recruiting | Rectal Cancer | Cyclophosphamide (CPA), Tecemotide (L-BLP25) | Phase 2 | 124 | Merck KGaA |
| NCT00828009 BLP25 Liposome Vaccine and Bevacizumab After Chemotherapy and Radiation Therapy in Treating Patients With Newly Diagnosed Stage IIIA or Stage IIIB Non-Small Cell Lung Cancer That Cannot Be Removed by Surgery | CDR0000632611, E6508 | December 2010 | January 2016 | Recruiting | Lung Cancer | Biological: bevacizumab, Biological: emepepimut-S, Drug: carboplatin, Drug: cyclophosphamide, Drug: paclitaxel, Radiation: radiation therapy | Phase 2 | 55 | Eastern Cooperative Oncology Group, National Cancer Institute (NCI) |
| NCT01462513 L-BLP25 in Patients With Colorectal Carcinoma After Curative Resection of Hepatic Metastases | LICC01 | August 2011 | September 2016 | Recruiting | Colon Carcinoma, Rectum Carcinoma | Biological: L-BLP25, Biological: Placebo | Phase 2 | 159 | Johannes Gutenberg University Mainz, Dr. Carl Schimanski |
| NCT01496131 Tecemotide (L-BLP25) in Prostate Cancer | EMR 63325-015, BB-IND 7787 | October 2011 | August 2017 | Recruiting | Prostate Cancer | Radiation: Radiation therapy, Drug: Goserelin, Drug: Cyclophosphamide, Drug: Tecemotide (L-BLP25) | Phase 2 | 48 | EMD Serono, National Cancer Institute (NCI) |
| NCT02049151 Tecemotide Following Concurrent Chemo-radiotherapy for Non-small Cell Lung Cancer | START2, EMR 63325-021, 2013-003760-30 | March 2014 | July 2018 | Recruiting | Carcinoma, Non-Small-Cell Lung | Drug: Tecemotide, Drug: Placebo, Drug: Cyclophosphamide (CPA), Drug: Saline (sodium chloride) | Phase 3 | 1002 | EMD Serono |
| NCT01423760 Collect Long-term Data on Subjects Following Participation in Previous EMD 531444 (L-BLP25 or BLP25 Liposome Vaccine) Clinical Trials | EMR 63325-011 | January 2012 | December 2019 | Enrolling by invitation | Non-Small Cell Lung Cancer, Multiple Myeloma | Biological: Tecemotide, Other: No intervention | 262 | Merck KGaA | |
| NCT01015443 Cancer Vaccine Study for Stage III, Unresectable, Non-small Cell Lung Cancer (NSCLC) in the Asian Population | INSPIRE, EMR63325-012 | December 2009 | May 2020 | Recruiting | Non-Small Cell Lung Cancer | Biological: Tecemotide (L-BLP25), Biological: Placebo | Phase 3 | 500 | Merck KGaA |
| Note: Merck KGaA and Oncothyreon are not reporting in their annual reports on colorectal cancer trials and prostate cancer trials[23][24] | |||||||||
Overview of completed trials
Overview of completed tecemotide trials (as of September 2, 2014)[25] where results have been published, sorted by Primary Completion Date:[26]
| ID | Phase | Indication | Start | Primary Completion Date | Summary of the results[27] |
|---|---|---|---|---|---|
| EMR 63325-005 | 2 | NSCLC | August 2000 | March 2006 | Subgroup analysis favorable |
| START, EMR 63325-001 | 3 | NSCLC | January 2007 | August 2012 | Primary endpoint not met. Subgroup analysis favorable |
| EMR 63325-009 (Japan study) | 1, 2 | NSCLC | December 2008 | May 2014 | Primary endpoint and secondary endpoints not met. Subgroup analysis not favorable |
Merck KGaA discontinues the development of tecemotide in NSCLC (Non-small cell lung cancer)
On August 18, 2014, Oncothyreon[28] and finally on September 12, 2014, also Merck KGaA informed that a randomized Phase 1/2 study, EMR 63325-009, of tecemotide compared to placebo in Japanese patients with Stage III non-small cell lung cancer did not meet its primary endpoint of an improvement in overall survival, and no treatment effect was seen in any of the secondary endpoints (progression free survival, time to progression or time to failure). Merck made the recommendation to stop the investigational treatment of patients in the EMR 63325-009 study in Japan.
Furthermore, Merck KGaA announced its decision to discontinue the Phase III START2 and INSPIRE studies, and all other Merck-sponsored clinical trials with tecemotide in NSCLC worldwide. Merck will continue to supply tecemotide for ongoing investigator-sponsored trials in other indications in accordance with their agreements with the sponsors of these studies.[29]
It remains unclear from Merck’s press release what happens with:
- the supply of ongoing investigator-sponsored NSCLC trials (non-monotherapy studies)
- Merck’s own tecemotide trials in indications other than NSCLC (prostate cancer, colorectal cancer)
Drug development risks
Risks that could affect the further development of tecemotide published in the annual reports of Oncothyreon (grantor of the license) and Merck KGaA (license holder; responsible for clinical development, marketing and manufacturing):
Risks related to efficacy
As published so far, primary end points have not been met in the clinical studies and tecemotide has shown only treatment effects in statistical analyses of certain subgroups.[30]
Risks related to patent situation
Oncothyreon’s patent protection for tecemotide in the U.S. will expire in 2018.[31]
Risks related to human resources
Merck KGaA is reporting problems with recruiting and retaining qualified employees: "Sourcing, recruiting and retaining specialists and talent at Merck are among the company’s top priorities. Nevertheless, employee-related risks that affect business activities are likely, even though their impact is difficult to assess. Merck rates this as a medium risk."[32]
Merck KGaA is further reporting with respect to its pharma division Merck Serono: "Over 80 % of the Merck Serono senior management positions replaced since 2011 [until Sept. 2014]."[33]
Risks related to novel technologies
Tecemotide is based on novel technologies, which may raise new regulatory issues that could delay or make regulatory approval more difficult. Additionally, to date, the FDA has approved for commercial sale in the United States only one active vaccine designed to stimulate an immune response against cancer. Consequently, there is limited precedent for the successful development or commercialization of products based on these technologies in this area.[34]
Risks related to manufacture
Merck KGaA currently relies on third-party manufacturers to supply the product candidate: On Baxter International Inc. (Baxter), for the manufacture of tecemotide, and on GlaxoSmithKline plc (GSK) for the manufacture of the adjuvant in tecemotide called monophosphoryl lipid A (MPL). If tecemotide is not approved until 2015, GSK may terminate its obligation to supply the adjuvant MPL. In this case, Oncothyreon would retain the necessary licenses from GSK required to have the adjuvant MPL manufactured, but the transfer of the process to a third party would delay the development and commercialization of tecemotide.[35]
GSK is developing the MAGE A3 vaccine in Phase 3, a direct competitor to tecemotide (see section below).
Risks related to competition
Competition in NSCLC: There are currently two products approved as maintenance therapy following treatment of inoperable locoregional Stage III NSCLC with induction chemotherapy, Tarceva (erlotinib), a targeted small molecule from Genentech, Inc., a member of the Roche Group, and Alimta (pemetrexed), a chemotherapeutic from Eli Lilly and Company. Tecemotide has not been tested in combination with or in comparison to these products. It is possible that other existing or new agents will be approved for this indication. In addition, there are at least two vaccines in development for the treatment of NSCLC, including GSK’s MAGE A3 vaccine in Phase 3 and Transgene’s TG-4010 in Phase 2/3. TG-4010 also targets MUC1, although using technology different from tecemotide.[36]
Drug development cost
The cost spent for the tecemotide development – beginning in the late 1990s – have not been published in detail by the companies Biomira/Oncothyreon, Merck KGaA and Ono Pharmaceutical. Additionally, the estimation of full cost of bringing a new drug to market – from discovery through clinical trials to approval – is complex and controversial.
However, a cautious estimate of the tecmotide development cost spent until 2014 ranges from 300 to 500 million Euros (390 to 650 million US$; for more information see Drug development).
History
| Date | Event |
|---|---|
| May 1998 | Biomira files a BLP25 (tecemotide) patent[37] |
| May 2001 | Biomira licenses BLP25 (tecemotide) to Merck KGaA |
| Aug 2001 | Biomira publishes results of a Phase I study of the BLP25 (tecemotide)[38] |
| Mar 2006 | Results of the Phase IIb Study (EMR 63325-005): Subgroup analysis favorable |
| Aug 2007 | Merck KGaA acquires worldwide marketing rights for tecemotide from Oncothyreon and will be entirely responsible for the further clinical development of tecemotide |
| Sep 2007 | Biomira changes company name to Oncothyreon |
| Dec 2008 | Merck KGaA acquires manufacturing rights for tecemotide from Oncothyreon |
| Dec 2009 | INSPIRE study (EMR63325-012) started. Estimated primary completion date is May 2020 |
| Oct 2011 | Ono Pharmaceutical acquires a co-development and co-marketing license for tecemotide in Japan |
| Dec 2012 | Results of the START study (EMR 63325-001): Primary endpoint not met. Subgroup analysis favorable |
| Mar 2014 | START2 study (EMR 63325-021) started. Estimated primary completion date is July 2018 |
| Aug 2014 | Results of the Japan study (EMR 63325-009): Primary endpoint and secondary endpoints not met. Subgroup analysis not favorable[39] |
| Sep 2014 | Merck KGaA terminates the NSCLC development |
References
- ↑ BLP25 liposome vaccine (Tecemotide) long-term safety results from Phase 2b study of patients with stage IIIB and IV non-small cell lung cancer (NSCLC) treated for 2 years or more; World Conference on Lung Cancer, August 2009
- ↑ Biomira announces plan to change name to Oncothyreon
- ↑ Aug 8, 2007, Biomira and Merck KGaA sign amended and restated collaboration and supply agreements related to Stimuvax
- ↑ Dec 18, 2008, Merck KGaA acquires manufacturing rights for Stimuvax from Oncothyreon
- ↑ Merck KGaA press release from October 4, 2011
- ↑ BLP25 liposome vaccine (Tecemotide) long-term safety results from Phase 2b study of patients with stage IIIB and IV non-small cell lung cancer (NSCLC) treated for 2 years or more; World Conference on Lung Cancer, August 2009
- ↑ WHO Drug Information, Vol. 26, No. 4, 2012; Proposed INN: List 108
- ↑ BLP25 liposome vaccine (Tecemotide) long-term safety results from Phase 2b study of patients with stage IIIB and IV non-small cell lung cancer (NSCLC) treated for 2 years or more; World Conference on Lung Cancer, August 2009
- ↑ BLP25 liposome vaccine (Tecemotide) long-term safety results from Phase 2b study of patients with stage IIIB and IV non-small cell lung cancer (NSCLC) treated for 2 years or more; World Conference on Lung Cancer, August 2009
- ↑ BLP25 liposome vaccine (Tecemotide) long-term safety results from Phase 2b study of patients with stage IIIB and IV non-small cell lung cancer (NSCLC) treated for 2 years or more; World Conference on Lung Cancer, August 2009
- ↑ ClinicalTrials.gov
- ↑ The Primary Completion Date is defined as the date when the final subject was examined or received an intervention for the purposes of final collection of data for the primary outcome.
- ↑ Butts, C. (2007) Stimuvax (L-BLP25): A Peptide Vaccine Strategy in Non-Small Cell Lung Cancer: M17-03. Journal of Thoracic Oncology 2, Supplement 4, S199–201. doi:10.1097/01.JTO.0000282977.65747.30
- ↑ Butts, C., Maksymiuk, A., Goss, G., Soulières, D., Marshall, E., Cormier, Y., Ellis, P.M., Price, A., Sawhney, R., Beier, F., Falk, M., Murray, N. (2011) Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): phase IIB randomized, multicenter, open-label trial. Journal of Cancer Research and Clinical Oncology 137, 1337–1342. doi:10.1007/s00432-011-1003-3
- ↑ Merck KGaA to test Stimuvax in breast cancer trial (FierceVaccines)
- ↑ UPDATE 2-Merck to test Stimuvax cancer drug in Phase III (Thomson Reuters)
- ↑ Merck KGaA press release from June 17, 2010
- ↑ Experimental Cancer Treatment L-BLP25 (Stimuvax®) Did Not Meet Primary Endpoint of Improvement in Overall Survival in Pivotal Phase III Trial in Patients with Non-Small Cell Lung Cancer (Onco'Zine - The International Cancer Network)
- ↑ Butts, C., Socinski, M.A., Mitchell, P.L., Thatcher, N., Havel, L., Krzakowski, M., Nawrocki, S., Ciuleanu, T.-E., Bosquée, L., Trigo, J.M., Spira, A., Tremblay, L., Nyman, J., Ramlau, R., Wickart-Johansson, G., Ellis, P., Gladkov, O., Pereira, J.R., Eberhardt, W.E.E., Helwig, C., Schröder, A., Shepherd, F.A. (2014) Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. The Lancet Oncology 15, 59–68. doi:10.1016/S1470-2045(13)70510-2
- ↑ Merck Discontinues Clinical Development Program of Tecemotide as a Monotherapy in Stage III Non-Small Cell Lung Cancer - Merck KGaA Press release from 12 Sept 2014
- ↑ Startschuss für innovative Brustkrebs-Impfstudie ABCSG-34
- ↑ ABCSG 34: Randomisierung beendet!
- ↑ Merck KGaA Annual Report 2013
- ↑ Oncothyreon Annual Report 2013 (Form 10-K)
- ↑ ClinicalTrials.gov
- ↑ The Primary Completion Date is defined as the date when the final subject was examined or received an intervention for the purposes of final collection of data for the primary outcome.
- ↑ All References related to this table can be found in the section "Overview and results of all trials"
- ↑ Oncothyreon's 8-K SEC filing from Aug 18, 2014
- ↑ Merck Discontinues Clinical Development Program of Tecemotide as a Monotherapy in Stage III Non-Small Cell Lung Cancer - Merck KGaA Press release from 12 Sept 2014
- ↑ Oncothyreon’s Annual Report 2013 (Form 10-K)
- ↑ Oncothyreon’s Annual Report 2013 (Form 10-K)
- ↑ Merck KGaA Annual Report 2013
- ↑ Merck Serono Investor & Analyst Day 2014 - Stefan Oschmann’s presentation - Slide 6 - 18 Sept 2014
- ↑ Oncothyreon’s Annual Report 2013 (Form 10-K)
- ↑ Oncothyreon’s Annual Report 2013 (Form 10-K)
- ↑ Oncothyreon’s Annual Report 2013 (Form 10-K)
- ↑ US Patent 6,600,012, Agrawal et al., Lipid-modified MUC-1 derivatives
- ↑ Palmer, M., Parker, J., Modi, S., Butts, C., Smylie, M., Meikle, A., Kehoe, M., MacLean, G., Longenecker, M. (2001) Phase I Study of the BLP25 (MUC1 Peptide) Liposomal Vaccine for Active Specific Immunotherapy in Stage IIIB/IV Non–Small-Cell Lung Cancer. Clinical Lung Cancer 3, 49–57. doi:10.3816/CLC.2001.n.018
- ↑ Oncothyreon's 8-K SEC filing from Aug 18, 2014