Tambjamine


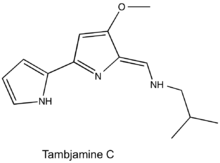
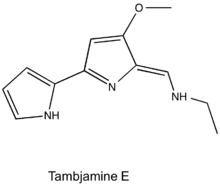
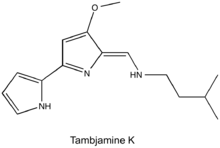
Tambjamines are a group of natural products that are structurally related to prodiginines and are characterized by a 4-methoxy-2,2'-bipyrrolenamine structure.[1] Tambjamines are composed of two pyrrole rings with an enamine moiety at C-5 and a methoxy group at C-4, and the majority have short alkyl chains substituted for the enamine nitrogen.[2] This group of alkaloids have been isolated from marine invertebrates and bacteria (both marine and terrestrial).
Marine sources and ecological roles
The large nudibranch Roboastra tigris is a known predator of Tambje eliora and Tambje abdere, two species of smaller nudibranchs. The chemical extracts of all three nudibranch species contain tambjamines, which were traced to Sessibugula translucens, a food source of the two prey species. It is hypothesized that tambjamines are a chemical defense mechanism of the bryozoan against feeding by the spotted kelpfish Gibbonsia elegans.[3][4]
Biosynthesis
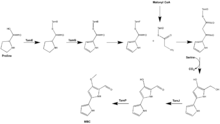

The biosynthetic gene cluster responsible for tambjamine production was identified in 2007 using functional genomic analysis of a Pseudoalteromonas tunicata strain. The Tam cluser consiste of 19 proteins, 12 of which were found to be highly similar to proteins in the Red and Pig pathways based on sequence data. The proposed biosynthesis of tambjamine YP1 involves the incorporation of proline, malonyl Co-A, and serine in the synthesis of MBC.
AfaA is hypothesized to activate long-chain fatty acids while the predicted dehydrogenase, TamT, indtroduces a double bond into a fatty acyl side chain. It is presumed that TamH then carries out the reduction of the CoA-ester to form an aldehyde intermediate, followed by transamination. Condensation of the dodec-3-en-1-amine product of this reaction and MBC by TamQ, results in the tambjamine YP1.[5]
References
- ↑ Iglesias Hernández, P; Moreno, D; Javier, A. A.; Torroba, T; Pérez-Tomás, R; Quesada, R (2012). "Tambjamine alkaloids and related synthetic analogs: Efficient transmembrane anion transporters". Chemical Communications. 48 (10): 1556–8. PMID 21528145. doi:10.1039/c1cc11300c.
- ↑ Carbone, M; Irace, C; Costagliola, F; Castelluccio, F; Villani, G; Calado, G; Padula, V; Cimino, G; Lucas Cervera, J; Santamaria, R; Gavagnin, M (2010). "A new cytotoxic tambjamine alkaloid from the Azorean nudibranch Tambja ceutae". Bioorganic & Medicinal Chemistry Letters. 20 (8): 2668–70. PMID 20227875. doi:10.1016/j.bmcl.2010.02.020.
- ↑ Carte, B.; Faulkner, D. J. (1983). "Defensive metabolites from three nembrothid nudibranchs". The Journal of Organic Chemistry. 48 (14): 2314. doi:10.1021/jo00162a003.
- ↑ Carté, B; Faulkner, D. J. (1986). "Role of secondary metabolites in feeding associations between a predatory nudibranch, two grazing nudibranchs, and a bryozoan". Journal of Chemical Ecology. 12 (3): 795–804. PMID 24306917. doi:10.1007/BF01012111.
- ↑ Burke, C; Thomas, T; Egan, S; Kjelleberg, S (2007). "The use of functional genomics for the identification of a gene cluster encoding for the biosynthesis of an antifungal tambjamine in the marine bacterium Pseudoalteromonas tunicata". Environmental Microbiology. 9 (3): 814–8. PMID 17298379. doi:10.1111/j.1462-2920.2006.01177.x.