TGF beta 1
Transforming growth factor beta 1 or TGF-β1 is a polypeptide member of the transforming growth factor beta superfamily of cytokines. It is a secreted protein that performs many cellular functions, including the control of cell growth, cell proliferation, cell differentiation and apoptosis. In humans, TGF-β1 is encoded by the TGFB1 gene.[5][6]
Function
TGF-β is a multifunctional set of peptides that controls proliferation, differentiation, and other functions in many cell types. TGF-β acts synergistically with TGFA in inducing transformation. It also acts as a negative autocrine growth factor. Dysregulation of TGF-β activation and signaling may result in apoptosis. Many cells synthesize TGF-β and almost all of them have specific receptors for this peptide. TGF-β1, TGF-β2, and TGF-β3 all function through the same receptor signaling systems.[7]
TGF-β1 was first identified in human platelets as a protein with a molecular mass of 25 kilodaltons with a potential role in wound healing.[8] It was later characterized as a large protein precursor (containing 390 amino acids) that was proteolytically processed to produce a mature peptide of 112 amino acids.[9]
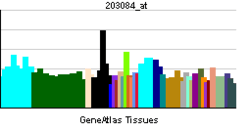
TGF-β1 plays an important role in controlling the immune system, and shows different activities on different types of cell, or cells at different developmental stages. Most immune cells (or leukocytes) secrete TGF-β1.[10]
T cells
Some T cells (e.g. regulatory T cells) release TGF-β1 to inhibit the actions of other T cells. Interleukin 1- and interleukin 2-dependent proliferation of activated T cells,[11][12] and the activation of quiescent helper T cells and cytotoxic T cells is prevented by the activity of TGF-β1.[13][14] Similarly, TGF-β1 can inhibit the secretion and activity of many other cytokines including interferon-γ, tumor necrosis factor-alpha (TNF-α) and various interleukins. It can also decrease the expression levels of cytokine receptors, such as the IL-2 receptor to down-regulate the activity of immune cells. However, TGF-β1 can also increase the expression of certain cytokines in T cells and promote their proliferation, particularly if the cells are immature.[10]
B cells
TGF-β1 has similar effects on B cells that also vary according to the differentiation state of the cell. It inhibits proliferation and stimulates apoptosis of B cells,[15] and plays a role in controlling the expression of antibody, transferrin and MHC class II proteins on immature and mature B cells.[10][15]
Myeloid cells
The effects of TGF-β1 on macrophages and monocytes is predominantly suppressive; this cytokine can inhibit the proliferation of these cells and prevent their production of reactive oxygen (e.g. superoxide (O2−)) and nitrogen (e.g. nitric oxide (NO)) intermediates. However, as with other cell types, TGF-β1 can also have the opposite effect on cells of myeloid origin. For example, TGF-β1 acts as a chemoattractant, directing an immune response to some pathogens; macrophages and monocytes respond to low levels of TGF-β1 in a chemotactic manner. Furthermore, the expression of monocytic cytokines (including interleukin-1(IL-1)-alpha, IL-1-beta, and TNF-α),[14] and phagocytic killing by macrophages can be increased by the action of TGF-β1.[10]
Interactions
TGF beta 1 has been shown to interact with:
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000105329 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000002603 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Ghadami M, Makita Y, Yoshida K, Nishimura G, Fukushima Y, Wakui K, Ikegawa S, Yamada K, Kondo S, Niikawa N, Tomita Ha (January 2000). "Genetic mapping of the Camurati-Engelmann disease locus to chromosome 19q13.1-q13.3". Am. J. Hum. Genet. 66 (1): 143–7. PMC 1288319
 . PMID 10631145. doi:10.1086/302728.
. PMID 10631145. doi:10.1086/302728. - ↑ Vaughn SP, Broussard S, Hall CR, Scott A, Blanton SH, Milunsky JM, Hecht JT (May 2000). "Confirmation of the mapping of the Camurati-Englemann locus to 19q13. 2 and refinement to a 3.2-cM region". Genomics. 66 (1): 119–21. PMID 10843814. doi:10.1006/geno.2000.6192.
- ↑ "Entrez Gene: TGFB1 transforming growth factor, beta 1".
- ↑ Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB (1983). "Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization". J. Biol. Chem. 258 (11): 7155–60. PMID 6602130.
- ↑ Derynck R, Jarrett JA, Chen EY, Eaton DH, Bell JR, Assoian RK, Roberts AB, Sporn MB, Goeddel DV (1985). "Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells". Nature. 316 (6030): 701–5. PMID 3861940. doi:10.1038/316701a0.
- 1 2 3 4 Letterio JJ, Roberts AB (1998). "Regulation of immune responses by TGF-beta". Annu. Rev. Immunol. 16: 137–61. PMID 9597127. doi:10.1146/annurev.immunol.16.1.137.
- ↑ Wahl SM, Hunt DA, Wong HL, Dougherty S, McCartney-Francis N, Wahl LM, Ellingsworth L, Schmidt JA, Hall G, Roberts AB (1988). "Transforming growth factor-beta is a potent immunosuppressive agent that inhibits IL-1-dependent lymphocyte proliferation". J. Immunol. 140 (9): 3026–32. PMID 3129508.
- ↑ Tiemessen MM, Kunzmann S, Schmidt-Weber CB, Garssen J, Bruijnzeel-Koomen CA, Knol EF, van Hoffen E (2003). "Transforming growth factor-beta inhibits human antigen-specific CD4+ T cell proliferation without modulating the cytokine response". Int. Immunol. 15 (12): 1495–504. PMID 14645158. doi:10.1093/intimm/dxg147.
- ↑ Gilbert KM, Thoman M, Bauche K, Pham T, Weigle WO (1997). "Transforming growth factor-beta 1 induces antigen-specific unresponsiveness in naive T cells". Immunol. Invest. 26 (4): 459–72. PMID 9246566. doi:10.3109/08820139709022702.
- 1 2 Wahl SM, Wen J, Moutsopoulos N (2006). "TGF-beta: a mobile purveyor of immune privilege". Immunol. Rev. 213: 213–27. PMID 16972906. doi:10.1111/j.1600-065X.2006.00437.x.
- 1 2 Lebman DA, Edmiston JS (1999). "The role of TGF-beta in growth, differentiation, and maturation of B lymphocytes". Microbes Infect. 1 (15): 1297–304. PMID 10611758. doi:10.1016/S1286-4579(99)00254-3.
- ↑ Hildebrand A, Romarís M, Rasmussen LM, Heinegård D, Twardzik DR, Border WA, Ruoslahti E (September 1994). "Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta". Biochem. J. 302 (2): 527–34. PMC 1137259
 . PMID 8093006. doi:10.1042/bj3020527.
. PMID 8093006. doi:10.1042/bj3020527. - ↑ Schönherr E, Broszat M, Brandan E, Bruckner P, Kresse H (July 1998). "Decorin core protein fragment Leu155-Val260 interacts with TGF-beta but does not compete for decorin binding to type I collagen". Arch. Biochem. Biophys. 355 (2): 241–8. PMID 9675033. doi:10.1006/abbi.1998.0720.
- ↑ Takeuchi Y, Kodama Y, Matsumoto T (Dec 1994). "Bone matrix decorin binds transforming growth factor-beta and enhances its bioactivity". J. Biol. Chem. 269 (51): 32634–8. PMID 7798269.
- ↑ Choy L, Derynck R (November 1998). "The type II transforming growth factor (TGF)-beta receptor-interacting protein TRIP-1 acts as a modulator of the TGF-beta response". J. Biol. Chem. 273 (47): 31455–62. PMID 9813058. doi:10.1074/jbc.273.47.31455.
- ↑ Saharinen J, Keski-Oja J (August 2000). "Specific sequence motif of 8-Cys repeats of TGF-beta binding proteins, LTBPs, creates a hydrophobic interaction surface for binding of small latent TGF-beta". Mol. Biol. Cell. 11 (8): 2691–704. PMC 14949
 . PMID 10930463. doi:10.1091/mbc.11.8.2691.
. PMID 10930463. doi:10.1091/mbc.11.8.2691. - ↑ Ebner R, Chen RH, Lawler S, Zioncheck T, Derynck R (November 1993). "Determination of type I receptor specificity by the type II receptors for TGF-beta or activin". Science. 262 (5135): 900–2. PMID 8235612. doi:10.1126/science.8235612.
- ↑ Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, Li E (March 2000). "Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis". Proc. Natl. Acad. Sci. U.S.A. 97 (6): 2626–31. PMC 15979
 . PMID 10716993. doi:10.1073/pnas.97.6.2626.
. PMID 10716993. doi:10.1073/pnas.97.6.2626. - ↑ McGonigle S, Beall MJ, Feeney EL, Pearce EJ (February 2001). "Conserved role for 14-3-3epsilon downstream of type I TGFbeta receptors". FEBS Lett. 490 (1-2): 65–9. PMID 11172812. doi:10.1016/s0014-5793(01)02133-0.
Further reading
- Border WA, Noble NA (1994). "Transforming growth factor beta in tissue fibrosis". N. Engl. J. Med. 331 (19): 1286–92. PMID 7935686. doi:10.1056/NEJM199411103311907.
- Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, Rifkin DB (1997). "Latent transforming growth factor-beta: structural features and mechanisms of activation". Kidney Int. 51 (5): 1376–82. PMID 9150447. doi:10.1038/ki.1997.188.
- Iozzo RV (1999). "The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins". J. Biol. Chem. 274 (27): 18843–6. PMID 10383378. doi:10.1074/jbc.274.27.18843.
- Reinhold D, Wrenger S, Kähne T, Ansorge S (1999). "HIV-1 Tat: immunosuppression via TGF-beta1 induction". Immunol. Today. 20 (8): 384–5. PMID 10431160. doi:10.1016/S0167-5699(99)01497-8.
- Yamada Y (2001). "Association of polymorphisms of the transforming growth factor-beta1 gene with genetic susceptibility to osteoporosis". Pharmacogenetics. 11 (9): 765–71. PMID 11740340. doi:10.1097/00008571-200112000-00004.
- Chen W, Wahl SM (2002). "TGF-beta: receptors, signaling pathways and autoimmunity". Curr. Dir. Autoimmun. Current Directions in Autoimmunity. 5: 62–91. ISBN 3-8055-7308-1. PMID 11826761. doi:10.1159/000060548.
- Marone M, Bonanno G, Rutella S, Leone G, Scambia G, Pierelli L (2002). "Survival and cell cycle control in early hematopoiesis: role of bcl-2, and the cyclin dependent kinase inhibitors P27 and P21". Leuk. Lymphoma. 43 (1): 51–7. PMID 11908736. doi:10.1080/10428190210195.
- Schnaper HW, Hayashida T, Hubchak SC, Poncelet AC (2003). "TGF-beta signal transduction and mesangial cell fibrogenesis". Am. J. Physiol. Renal Physiol. 284 (2): F243–52. PMID 12529270. doi:10.1152/ajprenal.00300.2002.
- Kalluri R, Neilson EG (2003). "Epithelial-mesenchymal transition and its implications for fibrosis". J. Clin. Invest. 112 (12): 1776–84. PMC 297008
 . PMID 14679171. doi:10.1172/JCI20530.
. PMID 14679171. doi:10.1172/JCI20530. - Grainger DJ (2004). "Transforming growth factor beta and atherosclerosis: so far, so good for the protective cytokine hypothesis". Arterioscler. Thromb. Vasc. Biol. 24 (3): 399–404. PMID 14699019. doi:10.1161/01.ATV.0000114567.76772.33.
- Attisano L, Labbé E (2004). "TGFbeta and Wnt pathway cross-talk". Cancer Metastasis Rev. 23 (1-2): 53–61. PMID 15000149. doi:10.1023/A:1025811012690.
- McGowan TA, Zhu Y, Sharma K (2004). "Transforming growth factor-beta: a clinical target for the treatment of diabetic nephropathy". Curr. Diab. Rep. 4 (6): 447–54. PMID 15539010. doi:10.1007/s11892-004-0055-z.
- Sheppard D (2005). "Integrin-mediated activation of latent transforming growth factor beta". Cancer Metastasis Rev. 24 (3): 395–402. PMID 16258727. doi:10.1007/s10555-005-5131-6.
- Gressner AM, Weiskirchen R (2006). "Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets". J. Cell. Mol. Med. 10 (1): 76–99. PMID 16563223. doi:10.1111/j.1582-4934.2006.tb00292.x.
- Seoane J (2006). "Escaping from the TGFbeta anti-proliferative control". Carcinogenesis. 27 (11): 2148–56. PMID 16698802. doi:10.1093/carcin/bgl068.
- Lee CG, Kang HR, Homer RJ, Chupp G, Elias JA (2006). "Transgenic modeling of transforming growth factor-beta(1): role of apoptosis in fibrosis and alveolar remodeling". Proc Am Thorac Soc. 3 (5): 418–23. PMC 2658706
 . PMID 16799085. doi:10.1513/pats.200602-017AW.
. PMID 16799085. doi:10.1513/pats.200602-017AW. - Wahl SM (2007). "Transforming growth factor-beta: innately bipolar". Curr. Opin. Immunol. 19 (1): 55–62. PMID 17137775. doi:10.1016/j.coi.2006.11.008.
- Redondo S, Santos-Gallego CG, Tejerina T (2007). "TGF-beta1: a novel target for cardiovascular pharmacology". Cytokine Growth Factor Rev. 18 (3-4): 279–86. PMID 17485238. doi:10.1016/j.cytogfr.2007.04.005.