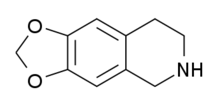
TDIQ
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C10H11NO2 |
| Molar mass | 177.199 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
TDIQ (also known as 6,7-methylenedioxy-1,2,3,4-tetrahydroisoquinoline or MDTHIQ) is a drug used in scientific research, which has anxiolytic and anorectic effects in animals.[1][2] It has an unusual effects profile in animals, with the effects generalising to cocaine and partially to MDMA and ephedrine,[3] but the effects did not generalise to amphetamine and TDIQ does not have any stimulant effects.[4] It is thought these effects are mediated via a partial agonist action at Alpha-2 adrenergic receptors, and TDIQ has been suggested as a possible drug for the treatment of cocaine dependence.[5]
See also
References
- ↑ Young R, Rothman RB, Rangisetty JB, Partilla JS, Dukat M, Glennon RA (May 2006). "TDIQ (5,6,7,8-tetrahydro-1,3-dioxolo[4,5-g]isoquinoline) inhibits the consumption of "snacks" in mice". Pharmacology, Biochemistry, and Behavior. 84 (1): 74–83. PMID 16750261. doi:10.1016/j.pbb.2006.04.007.
- ↑ Young R, Batkai S, Dukat M, Glennon RA (May 2006). "TDIQ (5,6,7,8-tetrahydro-1,3-dioxolo[4,5-g]isoquinoline) exhibits anxiolytic-like activity in a marble-burying assay in mice". Pharmacology, Biochemistry, and Behavior. 84 (1): 62–73. PMID 16750844. doi:10.1016/j.pbb.2006.04.006.
- ↑ Young R, Glennon RA (2002). "The stimulus effect of 5,6,7,8-tetrahydro-1,3-dioxolo[4,5-g]isoquinoline is similar to that of cocaine but different from that of amphetamine". Pharmacology, Biochemistry, and Behavior. 71 (1–2): 205–13. PMID 11812524. doi:10.1016/S0091-3057(01)00666-9.
- ↑ Glennon RA, Young R, Rangisetty JB (May 2002). "Further characterization of the stimulus properties of 5,6,7,8-tetrahydro-1,3-dioxolo[4,5-g]isoquinoline". Pharmacology, Biochemistry, and Behavior. 72 (1–2): 379–87. PMID 11900809. doi:10.1016/S0091-3057(01)00768-7.
- ↑ Young R (2007). "TDIQ (5,6,7,8-tetrahydro-1,3-dioxolo [4,5-g]isoquinoline): discovery, pharmacological effects, and therapeutic potential". CNS Drug Reviews. 13 (4): 405–22. PMID 18078426. doi:10.1111/j.1527-3458.2007.00022.x.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.