Acetone peroxide
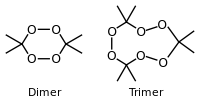 Cyclic dimer and trimer examples | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
3,3-Dimethyl-1,2-dioxacyclopropane (monomer) 3,3,6,6-Tetramethyl-1,2,4,5-tetraoxane (dimer) 3,3,6,6,9,9-Hexamethyl- 3,3,6,6,9,9,12,12-Octamethyl- | |||
| Other names
Triacetone triperoxide Peroxyacetone Mother of Satan | |||
| Identifiers | |||
| 3D model (JSmol) |
| ||
| ChemSpider | |||
| E number | E929 (glazing agents, ...) | ||
| PubChem CID |
|||
| |||
| |||
| Properties | |||
| C6H12O4 (dimer) C9H18O6 (trimer) C12H24O8 (tetramer) | |||
| Molar mass | 148.157 g/mol (dimer) 222.24 g/mol (trimer) | ||
| Appearance | White crystalline solid | ||
| Melting point | 131.5 to 133 °C (dimer)[1] 91 °C (trimer) | ||
| Boiling point | 97 to 160 °C (207 to 320 °F; 370 to 433 K) | ||
| Insoluble | |||
| Hazards | |||
| Main hazards | Explosive | ||
| Explosive data | |||
| Shock sensitivity | High / moderate when wet | ||
| Friction sensitivity | High / moderate when wet | ||
| Detonation velocity | 5300 m/s 17,384 ft/s 3.29 miles per second | ||
| RE factor | 0.83 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Acetone peroxide is an organic peroxide and a primary high explosive. It is produced by the oxidation of acetone to yield a mixture of linear monomer and cyclic dimer, trimer, and tetramer forms. The trimer is known as triacetone triperoxide (TATP) or tri-cyclic acetone peroxide (TCAP). Acetone peroxide takes the form of a white crystalline powder with a distinctive bleach-like odor and can explode if subjected to heat, friction, or shock. As a non-nitrogenous explosive, TATP has historically been more difficult to detect, and it has been implicated as the explosive used in terrorist attacks in Europe (2016 Brussels bombings) in 2017 and earlier.
History
Acetone peroxide (specifically, triacetone triperoxide) was discovered in 1895 by Richard Wolffenstein.[2] Wolffenstein combined acetone and hydrogen peroxide, and then he allowed the mixture to stand for a week at room temperature, during which time a small quantity of crystals precipitated, which had a melting point of 97 °C.[3]
In 1899 Adolf von Baeyer and Victor Villiger described the first synthesis of the dimer and described use of acids for the synthesis of both peroxides.[4] Baeyer and Villiger prepared the dimer by combining potassium persulfate in diethyl ether with acetone, under cooling. After separating the ether layer, the product was purified and found to melt at 132–133 °C.[5] They found that the trimer could be prepared by adding hydrochloric acid to a chilled mixture of acetone and hydrogen peroxide.[6] By using the depression of freezing points to determine the molecular weights of the compounds, they also determined that the form of acetone peroxide that they had prepared via potassium persulfate was a dimer, whereas the acetone peroxide that had been prepared via hydrochloric acid was a trimer, like Wolffenstein's compound.[7]
Work on this methodology and on the various products obtained, was further investigated in the mid-20th century by Milas and Golubović.[8]
Chemistry
The chemical name acetone peroxide is most commonly used to refer to the cyclic trimer, the product of a reaction between hydrogen peroxide and acetone in an acid-catalyzed nucleophilic addition, although various further monomeric and dimeric forms are possible.

Specifically, two dimers, one cyclic (C6H12O4) and one open chain (C6H14O4), as well as an open chain monomer (C3H8O4),[9] can also be formed; under a particular set of conditions of reagent and acid catalyst concentration, the cyclic trimer is the primary product.[8] A tetrameric form has also been described, under different catalytic conditions.[10] Under neutral conditions, the reaction is reported to produce the monomeric organic peroxide.[8]
Organic peroxides in general are sensitive, dangerous explosives, and all forms of acetone peroxide are sensitive to initiation. TATP decomposes explosively; examination of the explosive decomposition of TATP predicts "formation of acetone and ozone as the main decomposition products and not the intuitively expected oxidation products."[11] Very little heat is created by the explosive decomposition of TATP; the foregoing computational analysis suggests that TATP decomposition as an entropic explosion.[11] However, this hypothesis has been challenged as not conforming to actual measurements.[12] The tetrameric form of acetone peroxide, prepared under neutral conditions using a tin catalyst in the presence of a chelator or general inhibitor of radical chemistry, is reported to be more chemically stable, although still a very dangerous primary explosive.[10]
Some forms of acetone peroxide are prone to loss by sublimation and evaporation.
Several methods can be used for trace analysis of TATP,[13] including gas chromatography/mass spectrometry (GC/MS)[14][15][16][17][18] high performance liquid chromatography/mass spectrometry (HPLC/MS),[19][20][21][22][23] and HPLC with post-column derivitization.[24]
Industrial uses
Ketone peroxides, including acetone peroxide and methyl ethyl ketone peroxide, find application as initiators for polymerization reactions, e.g., silicone or polyester resins, in the making of fiberglass-reinforced composites. For these uses, the peroxides are typically in the form of a dilute solution in an organic solvent; methyl ethyl ketone is more common for this purpose, as it is stable in storage.
Acetone peroxides are common and unwanted by-products of oxidation reactions, such as those used in phenol syntheses.
Acetone peroxide is used as a flour bleaching agent to bleach and "mature" flour.[25]
Due to their explosive nature, their presence in chemical processes creates potential hazardous situations. Numerous methods are used to reduce their appearance as byproducts—for instance, shifting pH to more alkaline, adjusting reaction temperature, or adding inhibitors of their production.[26]
Use in improvised explosive devices
TATP and the other explosive forms of acetone peroxide belong to the few high explosives that do not contain nitrogen,[27] and so can pass undetected through explosive detection scanners designed to detect nitrogenous explosives.[28] Because of its high susceptibility to accidental detonation (and resulting "workplace accidents" in bomb-making shops), TATP has been referred to as the "Mother of Satan."[27] It is used by terrorists for its ability to evade detection aimed at nitrogenous explosives, and due to its low cost and the ease with which its precursors can be obtained.[27] It has been described in popular media as easily prepared from readily available retail ingredients, such as hair bleach and nail polish remover.[29]
TATP has been used in bomb and suicide attacks and in improvised explosive devices, including the London bombings on 7 July 2005, where four suicide bombers killed 52 people and injured more than 700.[30][31] It was one of the explosives used by the "shoe bomber" Richard Reid[31] and was used by the suicide bombers in the November 2015 Paris attacks,[29] 2016 Brussels bombings,[32] and 2017 Manchester Arena bombing.[33]
References
- ↑ Federoff, Basil T. et al., Encyclopedia of Explosives and Related Items (Springfield, Virginia: National Technical Information Service, 1960), vol. 1, p. A41.
- ↑ See:
- Wolffenstein R (1895). "Über die Einwirkung von Wasserstoffsuperoxyd auf Aceton und Mesityloxyd" [On the effect of hydrogen peroxide on acetone and mesityl oxide]. Berichte der Deutschen Chemischen Gesellschaft (in German). 28 (2): 2265–2269. doi:10.1002/cber.189502802208. Wolffenstein determined that acetone peroxide formed a trimer, and he proposed a structural formula for it. From pp. 2266–2267: "Die physikalischen Eigenschaften des Superoxyds, der feste Aggregatzustand, die Unlöslichkeit in Wasser etc. sprachen dafür, dass das Molekulargewicht desselben ein grösseres wäre, als dem einfachen Atomverhältnisse entsprach. … Es lag also ein trimolekulares Acetonsuperoxyd vor, das aus dem monomolekularen entstehen kann, indem sich die Bindungen zwischen je zwei Sauerstoffatomen lösen und zur Verknüpfung mit den Sauerstoffatomen eines benachbarten Moleküls dienen. Man gelangt so zur folgenden Constitutionsformel: [diagram of proposed molecular structure of the trimer of acetone peroxide] . Diese eigenthümliche ringförmig constituirte Verbindung soll Tri-Cycloacetonsuperoxyd genannt werden." (The physical properties of the peroxide, its solid state of aggregation, its insolubility in water, etc., suggested that its molecular weight would be a greater [one] than corresponded to its simple empirical formula. … Thus [the result of the molecular weight determination showed that] there was present a tri-molecular acetone peroxide, which can arise from the monomer by the bonds between each pair of oxygen atoms [on one molecule of acetone peroxide] breaking and serving as links to the oxygen atoms of a neighboring molecule. One thus arrives at the following structural formula: [diagram of proposed molecular structure of the trimer of acetone peroxide] . This strange ring-shaped compound shall be named "tri-cycloacetone peroxide".)
- Wolfenstein R (1895) Deutsches Reichspatent 84,953
- Matyáš R, Pachman J (2013). Primary Explosives. Berlin: Springer. p. 262. ISBN 978-3-642-28436-6.
- ↑ (Wolffenstein, 1895), p. 2266.
- ↑ See:
- Baeyer, Adolf and Villiger, Victor (1899) "Einwirkung des Caro'schen Reagens auf Ketone" (Effect of Caro's reagent on ketones [part 1]), Berichte der deutschen chemischen Gesellschaft, 32 : 3625–3633, see p. 3632.
- Baeyer A, Villiger V (1900). "Über die Einwirkung des Caro'schen Reagens auf Ketone" [On the effect of Caro's reagent on ketones [part 3]]. Berichte der deutschen chemischen Gesellschaft. 33 (1): 858–864. doi:10.1002/cber.190003301153.
- Baeyer A, Villiger V (1900). "Über die Nomenclatur der Superoxyde und die Superoxyde der Aldehyde" [On the nomenclature of peroxides and the peroxide of aldehydes]. Berichte der deutschen chemischen Gesellschaft. 33 (2): 2479–2487. doi:10.1002/cber.190003302185.
- Federoff, Basil T. et al., Encyclopedia of Explosives and Related Items (Springfield, Virginia: National Technical Information Service, 1960), vol. 1, p. A41.
- Matyáš, Robert and Pachman, Jirí, ed.s, Primary Explosives (Berlin, Germany: Springer, 2013), p. 257.
- ↑ (Baeyer and Villiger, 1899), p. 3632.
- ↑ (Baeyer and Villiger, 1900), p. 859.
- ↑ (Baeyer and Villiger, 1900), p. 859. From p. 859: "Das mit dem Caro'schen Reagens dargestellte, bei 132–133° schmelzende Superoxyd gab bei der Molekulargewichtsbestimmung nach der Gefrierpunktsmethode Resultate, welche zeigen, dass es dimolekular ist. Um zu sehen, ob das mit Salzsäure dargestellte Superoxyd vom Schmp. 90–94° mit dem Wolffenstein'schen identisch ist, wurde davon ebenfalls eine Molekulargewichtsbestimmung gemacht, welche auf Zahlen führte, die für ein trimolekulares Superoxyd stimmen." (The peroxide that was prepared with Caro's reagent and that melted at 132–133°C gave — according to a determination of molecular weight via the freezing point method — results which show that it is dimolecular. In order to see whether the peroxide that was prepared with hydrochloric acid and that has a melting point of 90–94°C is identical to Wolffenstein's, a molecular weight determination of it was likewise made, which led to numbers that are correct for a trimolecular peroxide.)
- 1 2 3 Milas NA, Golubović A (1959). "Studies in Organic Peroxides. XXVI. Organic Peroxides Derived from Acetone and Hydrogen Peroxide". Journal of the American Chemical Society. 81 (24): 6461–6462. doi:10.1021/ja01533a033.
- ↑ This is not the DMDO monomer referred to in the Chembox, but rather the open chain, dihydro monomer described by Milas & Goluboviç, op. cit.
- 1 2 Jiang H, Chu G, Gong H, Qiao Q (1999). "Tin Chloride Catalysed Oxidation of Acetone with Hydrogen Peroxide to Tetrameric Acetone Peroxide". Journal of Chemical Research. 28 (4): 288–289. doi:10.1039/a809955c.
- 1 2 Dubnikova F, Kosloff R, Almog J, Zeiri Y, Boese R, Itzhaky H, Alt A, Keinan E (Feb 2005). "Decomposition of triacetone triperoxide is an entropic explosion" (PDF). Journal of the American Chemical Society. 127 (4): 1146–59. PMID 15669854. doi:10.1021/ja0464903. Archived from the original (PDF) on 30 August 2006.
- ↑ Sinditskii VP, Koltsov VI, Egorshev, VY, Patrikeev DI, Dorofeeva OV (2014). "Thermochemistry of cyclic acetone peroxides". Thermochimica Acta. 585: 10–15. doi:10.1016/j.tca.2014.03.046.
- ↑ Schulte-Ladbeck R, Vogel M, Karst U (Oct 2006). "Recent methods for the determination of peroxide-based explosives". Analytical and Bioanalytical Chemistry. 386 (3): 559–65. PMID 16862379. doi:10.1007/s00216-006-0579-y.
- ↑ Muller D, Levy A, Shelef R, Abramovich-Bar S, Sonenfeld D, Tamiri T (Sep 2004). "Improved method for the detection of TATP after explosion". Journal of Forensic Sciences. 49 (5): 935–8. PMID 15461093.
- ↑ Stambouli A, El Bouri A, Bouayoun T, Bellimam MA (Dec 2004). "Headspace-GC/MS detection of TATP traces in post-explosion debris". Forensic Science International. 146 Suppl: S191–4. PMID 15639574. doi:10.1016/j.forsciint.2004.09.060.
- ↑ Oxley JC, Smith JL, Shinde K, Moran J (2005). "Determination of the Vapor Density of Triacetone Triperoxide (TATP) Using a Gas Chromatography Headspace Technique". Propellants, Explosives, Pyrotechnics. 30 (2): 127. doi:10.1002/prep.200400094.
- ↑ Sigman ME, Clark CD, Fidler R, Geiger CL, Clausen CA (2006). "Analysis of triacetone triperoxide by gas chromatography/mass spectrometry and gas chromatography/tandem mass spectrometry by electron and chemical ionization". Rapid Communications in Mass Spectrometry. 20 (19): 2851–7. PMID 16941533. doi:10.1002/rcm.2678.
- ↑ Romolo FS, Cassioli L, Grossi S, Cinelli G, Russo MV (Jan 2013). "Surface-sampling and analysis of TATP by swabbing and gas chromatography/mass spectrometry". Forensic Science International. 224 (1-3): 96–100. PMID 23219697. doi:10.1016/j.forsciint.2012.11.005.
- ↑ Widmer L, Watson S, Schlatter K, Crowson A (Dec 2002). "Development of an LC/MS method for the trace analysis of triacetone triperoxide (TATP)". The Analyst. 127 (12): 1627–32. PMID 12537371. doi:10.1039/B208350G.
- ↑ Xu X, van de Craats AM, Kok EM, de Bruyn PC (Nov 2004). "Trace analysis of peroxide explosives by high performance liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry (HPLC-APCI-MS/MS) for forensic applications". Journal of Forensic Sciences. 49 (6): 1230–6. PMID 15568694.
- ↑ Cotte-Rodríguez I, Hernandez-Soto H, Chen H, Cooks RG (Mar 2008). "In situ trace detection of peroxide explosives by desorption electrospray ionization and desorption atmospheric pressure chemical ionization". Analytical Chemistry. 80 (5): 1512–9. PMID 18247583. doi:10.1021/ac7020085.
- ↑ Sigman ME, Clark CD, Caiano T, Mullen R (2008). "Analysis of triacetone triperoxide (TATP) and TATP synthetic intermediates by electrospray ionization mass spectrometry". Rapid Communications in Mass Spectrometry. 22 (2): 84–90. PMID 18058960. doi:10.1002/rcm.3335.
- ↑ Sigman ME, Clark CD, Painter K, Milton C, Simatos E, Frisch JL, McCormick M, Bitter JL (Feb 2009). "Analysis of oligomeric peroxides in synthetic triacetone triperoxide samples by tandem mass spectrometry". Rapid Communications in Mass Spectrometry. 23 (3): 349–56. PMID 19125413. doi:10.1002/rcm.3879.
- ↑ Schulte-Ladbeck R, Kolla P, Karst U (Feb 2003). "Trace analysis of peroxide-based explosives". Analytical Chemistry. 75 (4): 731–5. PMID 12622359. doi:10.1021/ac020392n.
- ↑ Ferrari CG, Higashiuchi K, Podliska JA (1963). "Flour Maturing and Bleaching with Acyclic Acetone Peroxides" (PDF). Cereal Chemistry. 40: 89–100.
- ↑ Costantini, Michel (1991-03-26) Destruction of acetone peroxide. United States Patent 5003109. Freepatentsonline.com. Retrieved on 2013-02-03.
- 1 2 3 Glas K (2006-11-06). "TATP: Countering the Mother of Satan". The Future of Things. Retrieved 24 September 2009.
The tremendous devastative force of TATP, together with the relative ease of making it, as well as the difficulty in detecting it, made TATP one of the weapons of choice for terrorists
- ↑ "Feds are all wet on airport security". Star-Ledger (Newark, New Jersey). 2006-08-24. Retrieved 11 September 2009.
At the moment, Watts said, the screening devices are set to detect nitrogen-based explosives, a category that doesn't include TATP
- 1 2 Callimachi R, Rubin AJ, Fourquet L (2016-03-19). "A View of ISIS’s Evolution in New Details of Paris Attacks". The New York Times.
- ↑ Naughton P (2005-07-15). "TATP is suicide bombers' weapon of choice". The Times (UK). Archived from the original on 10 February 2008.
- 1 2 Vince G (15 July 2005). "Explosives linked to London bombings identified". New Scientist.
- ↑ ""La mère de Satan" ou TATP, l'explosif préféré de l'EI" ["Mother of Satan " or TATP , the preferred explosive of IE]. LeVif.be Express (in French).
- ↑ [https://www.theguardian.com/us-news/2017/may/25/paul-ryan-montana-gop-greg-gianforte-ben-jacobs]]
External links
| Wikimedia Commons has media related to Acetone peroxide. |