T-cell receptor
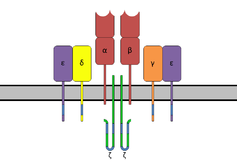 The T-cell receptor complex with TCR-α and TCR-β chains, CD3 and ζ-chain accessory molecules. | |||||||||
| Identifiers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | TCR_zetazeta | ||||||||
| Pfam | PF11628 | ||||||||
| InterPro | IPR021663 | ||||||||
| OPM superfamily | 261 | ||||||||
| OPM protein | 2hac | ||||||||
| |||||||||

| T-cell receptor alpha locus | |
|---|---|
| Identifiers | |
| Symbol | TRA |
| Alt. symbols | TCRA, TRA@ |
| Entrez | 6955 |
| HUGO | 12027 |
| OMIM | 186880 |
| Other data | |
| Locus | Chr. 14 q11.2 |
| T-cell receptor beta locus | |
|---|---|
| Identifiers | |
| Symbol | TRB |
| Alt. symbols | TCRB, TRB@ |
| Entrez | 6957 |
| HUGO | 12155 |
| OMIM | 186930 |
| Other data | |
| Locus | Chr. 7 q34 |
| T-cell receptor delta locus | |
|---|---|
| Identifiers | |
| Symbol | TRD |
| Alt. symbols | TCRD, TRD@, TCRDV1 |
| Entrez | 6964 |
| HUGO | 12252 |
| Other data | |
| Locus | Chr. 14 q11.2 |
| T-cell receptor gamma locus | |
|---|---|
| Identifiers | |
| Symbol | TRG |
| Alt. symbols | TCRG, TRG@ |
| Entrez | 6965 |
| HUGO | 12271 |
| Other data | |
| Locus | Chr. 7 p14 |
The T-cell receptor, or TCR, is a molecule found on the surface of T cells, or T lymphocytes,[1] that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding between TCR and antigen peptides is of relatively low affinity and is degenerate: that is, many TCRs recognize the same antigen peptide and many antigen peptides are recognized by the same TCR.[2]
The TCR is composed of two different protein chains (that is, it is a heterodimer). In humans, in 95% of T cells the TCR consists of an alpha (α) chain and a beta (β) chain (encoded by TRA and TRB, respectively), whereas in 5% of T cells the TCR consists of gamma and delta (γ/δ) chains (encoded by TRG and TRD, respectively). This ratio changes during ontogeny and in diseased states (such as leukemia). It also differs between species. Orthologues of the 4 loci have been mapped in various species.[3][4] Each locus can produce a variety of polypeptides with constant and variable regions.[3]
When the TCR engages with antigenic peptide and MHC (peptide/MHC), the T lymphocyte is activated through signal transduction, that is, a series of biochemical events mediated by associated enzymes, co-receptors, specialized adaptor molecules, and activated or released transcription factors.
History of the TCR
In 1983, Tak Wah Mak[5] and Mark M. Davis discovered the human and mouse TCR respectively. These findings allowed the entity and structure of the elusive TCR, known before as the "Holy Grail of Immunology", to be revealed. This allowed scientists from around the world to carry out studies on the TCR, leading to important studies in the fields of CAR-T, Cancer immunotherapy and Checkpoint inhibition.
Structural characteristics of the TCR
The TCR is a disulfide-linked membrane-anchored heterodimeric protein normally consisting of the highly variable alpha (α) and beta (β) chains expressed as part of a complex with the invariant CD3 chain molecules. T cells expressing this receptor are referred to as α:β (or αβ) T cells, though a minority of T cells express an alternate receptor, formed by variable gamma (γ) and delta (δ) chains, referred as γδ T cells.[6]
Each chain is composed of two extracellular domains: Variable (V) region and a Constant (C) region, both of Immunoglobulin superfamily (IgSF) domain forming antiparallel β-sheets. The Constant region is proximal to the cell membrane, followed by a transmembrane region and a short cytoplasmic tail, while the Variable region binds to the peptide/MHC complex.
The variable domain of both the TCR α-chain and β-chain each have three hypervariable or complementarity determining regions (CDRs). There is also an additional area of hypervariability on the β-chain (HV4) that does not normally contact antigen and, therefore, is not considered a CDR.
The residues in these variable domains are located in two regions of the TCR, at the interface of the α- and β-chains and in the β-chain framework region that is thought to be in proximity to the CD3 signal-transduction complex.[7] CDR3 is the main CDR responsible for recognizing processed antigen, although CDR1 of the alpha chain has also been shown to interact with the N-terminal part of the antigenic peptide, whereas CDR1 of the β-chain interacts with the C-terminal part of the peptide.
CDR2 is thought to recognize the MHC. CDR4 of the β-chain is not thought to participate in antigen recognition, but has been shown to interact with superantigens.
The constant domain of the TCR consists of short connecting sequences in which a cysteine residue forms disulfide bonds, which form a link between the two chains.
The TCR is a member of the immunoglobulin superfamily, a large group of proteins involved in binding, recognition, and adhesion; the family is named after antibodies (also called immunoglobulins). The TCR is similar to a half-antibody consisting of a single heavy and single light chain, except the heavy chain is without its crystallisable fraction (Fc). The two subunits of TCR are twisted together. Whereas the antibody uses its Fc region to bind to Fc Receptors on leukocytes, TCR is already docked onto the cell membrane. However, it is not able to mediate signal transduction itself due to its short cytoplasmic tail, so TCR still requires CD3 and zeta to carry out the signal transduction in its place, just as antibodies require binding to FcRs to initiate signal transduction. In this way the MHC-TCR-CD3 interaction for T cells is functionally similar to the antigen(Ag)-immunoglobulin(Ig)-FcR interaction for myeloid leukocytes, and Ag-Ig-CD79 interaction for B cells.
Generation of the TCR diversity
The generation of TCR diversity is similar to that for antibodies and B cell antigen receptors. It arises mainly from genetic recombination of the DNA encoded segments in individual somatic T cells by somatic V(D)J recombination using RAG1 and RAG2 recombinases. Unlike immunoglobulins, however, TCR genes do not undergo somatic hypermutation, and T cells do not express Activation-Induced (Cytidine) Deaminase (AID). The recombination process that creates diversity in BCR (antibodies) and TCR is unique to lymphocytes (T and B cells) during the early stages of their development in primary lymphoid organs (thymus for T cells, bone marrow for B cells).
Each recombined TCR possess unique antigen specificity, determined by the structure of the antigen-binding site formed by the α and β chains in case of αβ T cells or γ and δ chains on case of γδ T cells.[8]
- The TCR alpha chain is generated by VJ recombination, whereas the beta chain is generated by VDJ recombination (both involving a somewhat random joining of gene segments to generate the complete TCR chain).
- Likewise, generation of the TCR gamma chain involves VJ recombination, whereas generation of the TCR delta chain occurs by VDJ recombination.
The intersection of these specific regions (V and J for the alpha or gamma chain; V, D, and J for the beta or delta chain) corresponds to the CDR3 region that is important for peptide/MHC recognition (see above).
It is the unique combination of the segments at this region, along with palindromic and random nucleotide additions (respectively termed "P-" and "N-"), which accounts for the even greater diversity of T-cell receptor specificity for processed antigenic peptides.
Later during development, individual CDR loops of TCR can be re-edited in the periphery outside thymus by reactivation of recombinases using a process termed TCR revision (editing) and change its antigenic specificity.
The TCR complex
The TCR receptor complex is an octomeric complex of variable TCR receptor α and β chains with three dimeric signaling modules CD3δ/ε, CD3γ/ε and CD247 ζ/ζ or ζ/η. Ionizable residues in the transmembrane domain of each subunit form a polar network of interactions that hold the complex together.[9] Since the cytoplasmic tail of the TCR is extremely short, making it unlikely to participate in signaling, these signaling molecules are vital in propagating the signal from the triggered TCR into the cell.
Each T cell expresses clonal TCRs which recognize specific peptide/MHC complex during physical contact between T cell and antigen-presenting cell-APC (MHC class II) or any other cell type (MHC class I) [10] High on-rate and off-rate is characteristic for TCR and peptide/MHC interaction at physiological temperature. TCRs have very high degree of antigen specificity, despite of fact that the affinity to the peptide/MHC ligand is in the micromolar range.[11] This weak binding ( dissociation constant values) between TCR and peptide/MHC was determined by the surface plasmon resonance (SPR) to be in the range 1-100 μM, the association constant in the range from 1000 to 10000 M−1×s−1,[12] The TCR affinity for peptided/MHC has a direct impact on modulation of T-cell function. T cells are very sensitive to their antigens despite the low affinity of TCR for its peptide/MHC and low numbers of specific peptide/MHC on the surface of target cells.[13] The specific and efficient signaling via TCR might be regulated by dynamic oligomerization into TCR microclusters on the surface of T cells.[14] In this scenario, T-cell sensitivity to antigen could be increased via avidity-based mechanism. The antigen sensitivity is higher in antigen-experienced T cells than in naive T cells. Naive T cells pass through the process of functional avidity maturation with no change in affinity. It is based on the fact that effector and memory (antigen-experienced) T cell are less dependent on costimulatory signals and higher antigen concentration than naive T cell.[15]
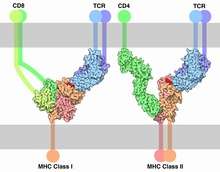
TCR co-receptors
The signal from the T-cell complex is enhanced by simultaneous binding of the MHC molecules by a specific co-receptor.
- On helper T cells and regulatory T cells, this co-receptor is CD4 that is specific for MHC class II.
- On cytotoxic T cells, this co-receptor is CD8 that is specific for MHC class I.
Extracellularly, the TCR co-receptor defines the specificity of the TCR to MHC class I or II molecule, and increases binding affinity of TCR to MHC to prolong the cell-cell interaction between the antigen-presenting cell and the T cell.
Intracellularly, the TCR co-receptor recruits essential molecules (e.g., LCK) involved in the signaling of the activated T lymphocyte to facilitate the CD3 signal transduction mechanism.
Associated molecules of the TCR complex involved in T-cell activation
The essential function of the TCR complex is to identify specific bound antigen and elicit a distinct and critical response. The signal transduction mechanism by which a T cell elicits this response upon contact with its unique antigen is termed T-cell activation (just as phototransduction is the term given to the signal transduction event by which photoreceptors elicits vision upon exposure to photons). There are myriad molecules involved in the complex biochemical process (called trans-membrane signaling) by which T-cell activation occurs.
The most common mechanism for activation and regulation of molecules beneath the lipid bilayer is via reversible tyrosine phosphorylation by protein kinase/phosphatase. T cells utilise the Src family kinases in transmembrane signalling largely to phosphorylate tyrosines that are part of immunoreceptor tyrosine-based activation motifs (ITAM) in intracellular parts of CD3 and ζ chains.[16]
Early signaling steps implicate the following kinases and phosphatases after TCR triggering:
- Lck – a Src family kinase associated with the intracellular tail of CD4 that phosphorylates CD3 and ζ ITAMs of the TCR complex
- FYN – a Src family kinase that phosphorylates CD3 and ζ ITAMs
- CD45 – a transmembrane protein whose intracellular tail functions as a tyrosine phosphatase that activates Src family kinases
- Zap70 – a Syk family kinase that binds to ITAM sequences upon tyrosine phosphorylation by Lck and Fyn, and phosphorylates LAT
When a T-cell receptor is activated by contact with a peptide:MHC complex, CD45 dephosphorylates inhibitory tyrosine of membrane-localized Src family kinases Fyn and Lck, previously recruited and activated by CD4 or CD8 coreceptors. Activated Fyn and Lck phosphorylates ITAMs on the CD3 and ζ chains. This allows cytoplasmic kinases of the Syk family (ZAP-70) to bind to the ITAM and activated ZAP-70 phosphorylates tyrosines on the adaptor protein LAT, which then attracts PLC-γ. Other downstream pathways are triggered as well (MAPK, NF-κB, NFAT) which results in gene transcription in the nucleus.[17]
See also
References
- ↑ Thomas J. Kindt; Richard A. Goldsby; Barbara Anne Osborne; Janis Kuby (2007). Kuby immunology. Macmillan. pp. 223–. ISBN 978-1-4292-0211-4. Retrieved 28 November 2010.
- ↑ Sewell, AK (2012), "Why must T cells be cross-reactive", Nat Rev Imm, 12 (9): 669–677, PMID 22918468
- 1 2 Glusman, G; et al. (2001), "Comparative genomics of the human and mouse T cell receptor loci", Immunity, 15 (3): 337–349, PMID 11567625, doi:10.1016/s1074-7613(01)00200-x.
- ↑ Deakin, JE; et al. (2006), "Physical mapping of T cell receptor loci (TRA@, TRB@, TRD@ and TRG@) in the opossum (Monodelphis domestica)", Cytogenet Genome Res, 112 (3–4): 342K, PMID 16484802, doi:10.1159/000089901.
- ↑ Yanagi, Yusuke; Yoshikai, Yasunobu; Leggett, Kathleen; Clark, Stephen P.; Aleksander, Ingrid; Mak, Tak W. (1984-03-08). "A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains". Nature. 308 (5955): 145–149. doi:10.1038/308145a0.
- ↑ Janeway CA Jr; Travers P; Walport M; et al. (2001). Immunobiology: The Immune System in Health and Disease. 5th edition. Glossary: Garland Science.
- ↑ Kieke MC, Shusta EV, Boder ET, Teyton L, Wittrup KD, Kranz DM (May 1999). "Selection of functional T cell receptor mutants from a yeast surface-display library". Proc. Natl. Acad. Sci. U.S.A. 96 (10): 5651–6. PMC 21915
 . PMID 10318939. doi:10.1073/pnas.96.10.5651.
. PMID 10318939. doi:10.1073/pnas.96.10.5651. - ↑ Janeway CA Jr; Travers P; Walport M; et al. (2001). Immunobiology: The Immune System in Health and Disease. 5th edition. Chapter 4, The Generation of Lymphocyte Antigen Receptors: Garland Science.
- ↑ Call ME, Pyrdol J, Wiedmann M, Wucherpfennig KW (December 2002). "The organizing principle in the formation of the T cell receptor-CD3 complex.". Cell. 111 (7): 967–79. PMC 3420808
 . PMID 12507424. doi:10.1016/s0092-8674(02)01194-7.
. PMID 12507424. doi:10.1016/s0092-8674(02)01194-7. - ↑ Smith-Garvin JE, Koretzky GA, Jordan MS (2009). "T cell activation". Annu. Rev. Immunol. 27: 591–619. PMC 2740335
 . PMID 19132916. doi:10.1146/annurev.immunol.021908.132706.
. PMID 19132916. doi:10.1146/annurev.immunol.021908.132706. - ↑ Donermeyer DL, Weber KS, Kranz DM, Allen PM (November 2006). "The study of high-affinity TCRs reveals duality in T cell recognition of antigen: specificity and degeneracy". J. Immunol. 177 (10): 6911–9. PMID 17082606. doi:10.4049/jimmunol.177.10.6911.
- ↑ Cole DK, Pumphrey NJ, Boulter JM, Sami M, Bell JI, Gostick E, Price DA, Gao GF, Sewell AK, Jakobsen BK (May 2007). "Human TCR-binding affinity is governed by MHC class restriction". J. Immunol. 178 (9): 5727–34. PMID 17442956. doi:10.4049/jimmunol.178.9.5727.
- ↑ Edwards LJ, Evavold BD (2011). "T cell recognition of weak ligands: roles of signaling, receptor number, and affinity.". Immunol Res. 50 (1): 39–48. PMC 3107861
 . PMID 21365321. doi:10.1007/s12026-011-8204-3.
. PMID 21365321. doi:10.1007/s12026-011-8204-3. - ↑ Schamel WW, Alarcón B (January 2013). "Organization of the resting TCR in nanoscale oligomers". Immunol. Rev. 251 (1): 13–20. PMID 23278737. doi:10.1111/imr.12019.
- ↑ von Essen MR, Kongsbak M, Geisler C (2012). "Mechanisms behind functional avidity maturation in T cells". Clin. Dev. Immunol. 2012: 163453. PMC 3351025
 . PMID 22611418. doi:10.1155/2012/163453.
. PMID 22611418. doi:10.1155/2012/163453. - ↑ Abram CL, Lowell CA (March 2007). "The expanding role for ITAM-based signaling pathways in immune cells". Sci. STKE. 2007 (377): re2. PMID 17356173. doi:10.1126/stke.3772007re2.
- ↑ Parham, Peter (2009). The Immune System. New York: Garland Science. pp. 22–223. ISBN 978-0-8153-4146-8.
External links
- T-cell Group – Cardiff University
- UMich Orientation of Proteins in Membranes protein/pdbid-2hac – Zeta-zeta dimer of T cell receptor
- T-Cell Receptor at the US National Library of Medicine Medical Subject Headings (MeSH)