Synaptobrevin
| Synaptobrevin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
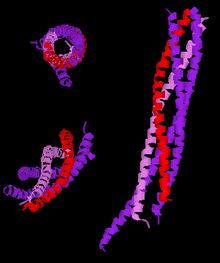 Three different views of the high resolution structure of a truncated neuronal SNARE complex. Legend: synaptobrevin-2 (red), Syntaxin-1 (pink), SNAP-25 (purple). | |||||||||
| Identifiers | |||||||||
| Symbol | Synaptobrevin | ||||||||
| Pfam | PF00957 | ||||||||
| InterPro | IPR001388 | ||||||||
| PROSITE | PDOC00368 | ||||||||
| SCOP | 1sfc | ||||||||
| SUPERFAMILY | 1sfc | ||||||||
| OPM superfamily | 218 | ||||||||
| OPM protein | 4wy4 | ||||||||
| |||||||||
Synaptobrevins (synaptobrevin isotypes 1-2) are small integral membrane proteins of secretory vesicles with molecular weight of 18 kilodalton (kDa) that are part of the vesicle-associated membrane protein (VAMP) family.[1][2][3][4][5]
Synaptobrevin is one of the SNARE proteins involved in formation of the SNARE complexes.
Structure
Out of four α-helices of the core SNARE complex one is contributed by synaptobrevin, one by syntaxin, and two by SNAP-25 (in neurons).
Function
SNARE proteins are the key components of the molecular machinery that drives fusion of membranes in exocytosis. Their function however is subject to fine-tuning by various regulatory proteins collectively referred to as SNARE masters.
Classification
In the Q/R nomenclature for organizing SNARE proteins, VAMP/synaptobrevin family members are classified as R-SNAREs, so named for the presence of an arginine at a specific location within the primary sequence of the protein (as opposed to the SNAREs of the target membrane, which contain a glutamine and are so named Q-SNAREs). Synaptobrevin is classified as a V-SNARE in the V/T nomenclature, an alternative classification scheme in which SNAREs are classified as V-SNAREs and T-SNAREs for their localization to vesicles and target membranes, respectively.[6]
Clinical significance
Synaptobrevin is degraded by tetanospasmin, a protein derived from the bacterium Clostridium tetani, which causes tetanus. A related bacterium, Clostridium botulinum, produces botulinum toxin that also hydrolyzes synaptobrevin.
Human proteins containing this domain
SEC22A; SEC22B; SYBL1; VAMP1; VAMP2; VAMP3; VAMP4; VAMP5; VAMP8; YKT6;
References and notes
- ↑ Baumert M, Maycox PR, Navone F, De Camilli P, Jahn R (February 1, 1989). "Synaptobrevin: an integral membrane protein of 18,000 daltons present in small synaptic vesicles of rat brain". EMBO J. 8 (2): 379–84. PMC 400817
 . PMID 2498078.
. PMID 2498078.
- ↑ Bock JB, Scheller RH (October 1999). "SNARE proteins mediate lipid bilayer fusion". Proc. Natl. Acad. Sci. U.S.A. 96 (22): 12227–9. PMC 34255
 . PMID 10535902. doi:10.1073/pnas.96.22.12227.
. PMID 10535902. doi:10.1073/pnas.96.22.12227.
- ↑ Ernst JA, Brunger AT (2003). "High resolution structure, stability, and synaptotagmin binding of a truncated neuronal SNARE complex". J Biol Chem. 278 (10): 8630–6. PMID 12496247. doi:10.1074/jbc.M211889200.
- ↑ Fasshauer D, Sutton RB, Brunger AT, Jahn R (December 1998). "Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs". Proc. Natl. Acad. Sci. U.S.A. 95 (26): 15781–6. PMC 28121
 . PMID 9861047. doi:10.1073/pnas.95.26.15781.
. PMID 9861047. doi:10.1073/pnas.95.26.15781.
- ↑ Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE (1998). "SNAREpins: minimal machinery for membrane fusion". Cell. 92 (6): 759–72. PMID 9529252. doi:10.1016/S0092-8674(00)81404-X.
- ↑ Juan S. Bonifacino and Benjamin S. Glick. "The Mechanisms of Vesicle Budding and Fusion." Cell, Vol. 116, 153–166, January 23, 2004,
External links
- Synaptobrevin at the US National Library of Medicine Medical Subject Headings (MeSH)