Sulfenyl chloride

A sulfenyl chloride is a functional group with the connectivity R–S–Cl, where R is alkyl[1] or aryl. Sulfenyl chlorides are reactive compounds that behave as sources of RS+. They are used in the formation of RS–N and RS–O bonds. According to IUPAC nomenclature they are named as alkyl thiohypochlorites, i.e. esters of thiohypochlorous acid.
Preparation
Sulfenyl chlorides are typically prepared by chlorination of disulfides:[2][3]
- R2S2 + Cl2 → 2 RSCl
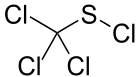
This reaction is sometimes called the Zincke disulfide reaction, in recognition of Theodor Zincke.[4][5] Typically, sulfenyl halides are stabilized by electronegative substituents. This trend is illustrated by the stability of CCl3SCl obtained by chlorination of carbon disulfide.
A general approach to the formation of sulfinyl chlorides, RS(O)Cl, is by reaction of the corresponding thiol with sulfuryl chloride, SO
2Cl
2, but in some cases the sulfenyl chloride results instead, as happens with 2,2,2-trifluoro-1,1-diphenylethanethiol. A trifluoroperacetic acid oxidation then provides a general approach to formation of sulfinyl chlorides from sulfenyl chlorides:[6]
Cl.png)
Reactions
CCl3SCl reacts with N-H-containing compounds in the presence of base to give the sulfenamides:
- CCl3SCl + R2NH → CCl3SNR2 + HCl
This method is used in the production of the fungicide captan.
Sulfenyl chlorides add across alkenes:[7]
- CH2=CH2 + RSCl → RSCH2CH2Cl
They undergo chlorination to the trichlorides:[3]
- CH3SCl + Cl2 → [CH3SCl2]Cl
Related compounds
Sulfenyl bromides are also known.[8] Simple sulfenyl iodides are unknown because they are unstable with respect to the disulfide and iodine:
- 2 RSI → (RS)2 + I2
Sulfenyl iodides can be isolated as stable compounds if they bear alkyl steric protecting groups as part of a cavity-shaped framework, illustrating the technique of kinetic stabilization of a reactive functionality, as in the case of sulfenic acids.[9]
A related class of compounds are the alkylsulfur trichlorides, as exemplified by methylsulfur trichloride, CH3SCl3.[10]
The corresponding selenenyl halides, e.g. C6H5SeCl, are more commonly encountered in the laboratory. Sulfenyl chlorides are used in the production of agents used in the vulcanization of rubber.
References
- ↑ Drabowicz, J.; Kiełbasiński, P.; Łyżwa, P.; Zając, A.; Mikołajczyk, M. (2008). Kambe, N., ed. Alkanesulfenyl Halides. Science of Synthesis. 39. pp. 544–550. ISBN 9781588905307.
- ↑ Hubacher, Max H. (1943). "o-Nitrophenylsulfur chloride". Org. Synth.; Coll. Vol., 2, p. 455
- 1 2 Douglass, Irwin B.; Norton, Richard V. (1973). "Methanesulfinyl Chloride". Org. Synth.; Coll. Vol., 5, pp. 709–715
- ↑ Zincke, Th. (1911). "Über eine neue Reihe aromatischer Schwefelverbindungen". Chemische Berichte (in German). 44 (1): 769–771. doi:10.1002/cber.191104401109.
- ↑ Zincke, Th.; Farr, Fr. (1912). "Über o-Nitrophenylschwefelchlorid und Umwandlungsprodukte". Justus Liebig's Annalen der Chemie (in German). 391 (1): 57–88. doi:10.1002/jlac.19123910106.
- ↑ Page, P. C. B.; Wilkes, R. D.; Reynolds, D. (1995). "Alkyl Chalcogenides: Sulfur-based Functional Groups". In Ley, Steven V. Synthesis: Carbon with One Heteroatom Attached by a Single Bond. Comprehensive Organic Functional Group Transformations. Elsevier. pp. 113–276. ISBN 9780080423234.
- ↑ Brintzinger, H.; Langheck, M., "Synthesen mit Alkylschwefelchloriden (X. Mitteil. über organische Schwefelchloride)", Chemische Berichte 1954, volume 87, 325-330. doi:10.1002/cber.19540870306
- ↑ Reno, Daniel S.; Pariza, Richard J. (1998). "Phenyl Vinyl Sulfide". Org. Synth.; Coll. Vol., 9, p. 662
- ↑ Sase, S.; Aoki, Y.; Abe, N.; Goto, K. (2009). "Stable Sulfenyl Iodide Bearing a Primary Alkyl Steric Protection Group with a Cavity-shaped Framework". Chemistry Letters. 38 (12): 1188–1189. doi:10.1246/cl.2009.1188.
- ↑ Braverman, S.; Cherkinsky, M.; Levinger, S. (2008). "Alkylsulfur Trihalides". Sci. Synth. 39: 187–188. ISBN 9781588905307.