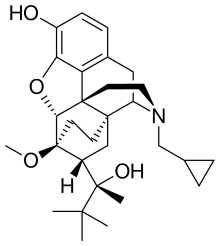
Buprenorphine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Suboxone (when combined with naloxone), Subutex, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605002 |
| Pregnancy category | |
| Routes of administration | Sublingual, buccal, IM, IV, transdermal, intranasal, rectally, by mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability |
Sublingual: 30%[1] Intranasal: 48%[2] |
| Protein binding | 96% |
| Metabolism | Hepatic (CYP3A4, CYP2C8) |
| Biological half-life | 37 hours (range 20–70 hours) |
| Excretion | Biliary and renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.052.664 |
| Chemical and physical data | |
| Formula | C29H41NO4 |
| Molar mass | 467.64 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Buprenorphine, sold under the brand name Subutex, among others, is an opioid used to treat opioid addiction, moderate acute pain and moderate chronic pain.[3] The combination buprenorphine/naloxone is also used for opioid addiction.
It is a semisynthetic derivative of thebaine. It is a mixed partial agonist opioid receptor modulator.
Buprenorphine was approved for medical use in the United States in 1981.[4]
Medical uses

Its primary uses in medicine are in the treatment of those addicted to opioids, such as heroin and oxycodone, but it may also be used to treat pain, and sometimes nausea in antiemetic intolerant individuals, most often in transdermal patch form.[3]
It has veterinary medical use for treatment of pain in dogs and cats.[5][6]
Opioid addiction
Buprenorphine versus methadone
Both buprenorphine and methadone are medications used for detoxification, short- and long-term opioid replacement therapy. Buprenorphine has the advantage of being only a partial agonist; hence negating the potential for life-threatening respiratory depression in cases of abuse.[7] Studies show the effectiveness of buprenorphine and methadone are almost identical, and largely share adverse-effect profiles apart from more sedation among methadone users. At low flexible doses from 2 to 6 mg, however, buprenorphine has a lower retention rate than from low doses 40 mg or less of methadone.[8]
Inpatient rehabilitation and detoxification
Rehabilitation programs consist of "detox" and "treatment" phases. The detoxification ("detox") phase consists of medically supervised withdrawal from the drug of dependency onto buprenorphine, sometimes aided by the use of medications such as benzodiazepines like oxazepam or diazepam (modern milder tranquilizers that assist with anxiety, sleep, and muscle relaxation), clonidine (a blood-pressure medication that may reduce some opioid withdrawal symptoms), and anti-inflammatory/pain relief drugs such as ibuprofen and aspirin.
The treatment phase begins once the person is stabilized and receives medical clearance. This portion of treatment consists of multiple therapy sessions, which include both group and individual counseling with various chemical dependency counselors, psychologists, psychiatrists, social workers, and other professionals. In addition, many treatment centers utilize twelve-step facilitation techniques, embracing the 12-step programs practiced by such organizations as Alcoholics Anonymous and Narcotics Anonymous. Some people on maintenance therapies have veered away from such organizations as Narcotics Anonymous, instead opting to create their own twelve-step fellowships (such as Methadone Anonymous) or depart entirely from the twelve-step model of recovery, seeking a program which is secular and based on science (such as SMART Recovery) rather than faith.[9][10]
Chronic pain relief
A transdermal patch is available for the treatment of chronic pain. It is not indicated for use in acute pain, pain that is expected to last only for a short period of time, or post-operative pain, nor is it indicated or recommended for use in the treatment of opioid addiction.[11]
Adverse effects

Common adverse drug reactions associated with the use of buprenorphine are similar to those of other opioids and include: nausea and vomiting, drowsiness, dizziness, headache, memory loss, cognitive and neural inhibition, perspiration, itchiness, dry mouth, shrinking of the pupils of the eyes (miosis), orthostatic hypotension, male ejaculatory difficulty, decreased libido, and urinary retention. Constipation and CNS effects are seen less frequently than with morphine.[13]
Buprenorphine dependence
Buprenorphine treatment carries the risk of causing psychological and or physical dependence. Buprenorphine has a slow onset, mild effect, and is very long acting with a half-life of 24 to 60 hours. Once a patient has stabilized on the drug, there are two treatment options; continual use/maintenance, or medically supervised withdrawal from the physical dependence to the drug.[14]
Respiratory effects
The most severe and serious adverse reaction associated with opioid use, in general, is respiratory depression, the mechanism behind fatal overdose.
Buprenorphine behaves differently than other opioids in this respect, as it shows a ceiling effect for respiratory depression.[13] Moreover, doubts about the antagonisation of the respiratory effects by naloxone have been disproved: Buprenorphine effects can be antagonised with a continuous infusion of naloxone.[15]
Concurrent use of buprenorphine with other CNS depressants (such as alcohol or benzodiazepines) is contraindicated as it may lead to fatal respiratory depression. Benzodiazepines, in recommended doses, are not contraindicated in individuals tolerant to either opioids or benzodiazepines.
Pain management
People on high-dose buprenorphine therapy may be unaffected by even large doses of opioids such as oxycodone, morphine, or hydromorphone.
It is also difficult to achieve acute opioid analgesia in persons using buprenorphine for opioid replacement therapy.[16]
Pharmacology
Pharmacodynamics
Buprenorphine has been reported to possess the following pharmacological activity:[17]
| Receptor | Action | Ki | Emax | Comments |
|---|---|---|---|---|
| MOR | Partial agonist | 1.5 nM | 28.7% | Binds with high affinity, but only partially activates the receptor. This behavior is responsible for buprenorphine's ability to block most μ agonists and the phenomenon of withdrawal effects when used in actively opioid dependent persons. |
| KOR | Antagonist | 2.5 nM | 0% | Possible therapeutic applications as animal models show antidepressive, anxiolytic, stress relieving, and anti-addictive properties with κ antagonists. |
| DOR | Antagonist | 6.1 nM | 0% | Possible attenuation of drug reward |
| NOP | Partial agonist | 77.4 nM | 15.5% | |
| TLR4 | Agonist | ?? | ?? |
In simplified terms, buprenorphine can essentially be thought of as a non-selective, mixed agonist–antagonist opioid receptor modulator,[18] acting as a weak partial agonist of the MOR, an antagonist of the KOR, an antagonist of the DOR, and a relatively low-affinity, very weak partial agonist of the ORL-1.[19][20][21][22][23][24]
Buprenorphine is also known to bind to with high affinity and antagonize the putative ε-opioid receptor.[25][26]
Unlike some other opioids and opioid antagonists, buprenorphine binds only weakly to and possesses little if any activity at the sigma receptor.[27][28]
Buprenorphine also blocks voltage-gated sodium channels via the local anesthetic binding site, and this underlies its potent local anesthetic properties.[29]
Similarly to various other opioids, buprenorphine has also been found to act as an agonist of the toll-like receptor 4.[30]
Full analgesic efficacy of buprenorphine requires both exon 11-[31] and exon 1-associated μ-opioid receptor splice variants.[32]
Pharmacokinetics
Buprenorphine is metabolised by the liver, via CYP3A4 (also CYP2C8 seems to be involved) isozymes of the cytochrome P450 enzyme system, into norbuprenorphine (by N-dealkylation). The glucuronidation of buprenorphine is primarily carried out by UGT1A1 and UGT2B7, and that of norbuprenorphine by UGT1A1 and UGT1A3. These glucuronides are then eliminated mainly through excretion into the bile. The elimination half-life of buprenorphine is 20–73 hours (mean 37). Due to the mainly hepatic elimination, there is no risk of accumulation in people with renal impairment.[33]
One of the major active metabolites of buprenorphine is norbuprenorphine, which, contrary to buprenorphine itself, is a full agonist of the MOR, DOR, and ORL-1, and a partial agonist at the KOR.[34][35] However, relative to buprenorphine, norbuprenorphine has extremely little antinociceptive potency (1/50th that of buprenorphine), but markedly depresses respiration (10-fold more than buprenorphine).[36] This can be explained by very poor brain penetration of norbuprenorphine due to a high affinity of the compound for P-glycoprotein.[36] In contrast to norbuprenorphine, buprenorphine and its glucuronide metabolites are negligibly transported by P-glycoprotein.[36]
The glucuronides of buprenorphine and norbuprenorphine are also biologically active, and represent major active metabolites of buprenorphine.[37] Buprenorphine-3-glucuronide has affinity for the MOR (Ki = 4.9 pM), DOR (Ki = 270 nM) and ORL-1 (Ki = 36 µM), and no affinity for the KOR. It has a small antinociceptive effect and no effect on respiration. Norbuprenorphine-3-glucuronide has no affinity for the MOR or DOR, but does bind to the KOR (Ki = 300 nM) and ORL-1 (Ki = 18 µM). It has a sedative effect but no effect on respiration.
Chemistry
Buprenorphine is a semi-synthetic analogue of thebaine[38] and is fairly soluble in water, as its hydrochloride salt.[7] It also degrades in the presence of light.[7]
Detection in body fluids
Buprenorphine and norbuprenorphine may be quantitated in blood or urine to monitor use or abuse, confirm a diagnosis of poisoning, or assist in a medicolegal investigation. There is a significant overlap of drug concentrations in body fluids within the possible spectrum of physiological reactions ranging from asymptomatic to comatose. Therefore, it is critical to have knowledge of both the route of administration of the drug and the level of tolerance to opioids of the individual when results are interpreted.[39]
History
In 1969, researchers at Reckitt & Colman (now Reckitt Benckiser) had spent 10 years attempting to synthesize an opioid compound "with structures substantially more complex than morphine [that] could retain the desirable actions whilst shedding the undesirable side effects". Physical dependence and withdrawal from buprenorphine itself, remains an issue because it is a long-acting opiate.[40] Reckitt found success when researchers synthesized RX6029 which had showed success in reducing dependence in test animals. RX6029 was named buprenorphine and began trials on humans in 1971.[41][42] By 1978, buprenorphine was first launched in the UK as an injection to treat severe pain, with a sublingual formulation released in 1982.
Society and culture
Regulation
In the United States, buprenorphine (Subutex) and buprenorphine with naloxone (Suboxone) were approved for opioid addiction by the United States Food and Drug Administration in October 2002.[43] The FDA rescheduled buprenorphine from a Schedule V drug to a Schedule III drug just before approval of Subutex and Suboxone. The ACSCN for buprenorphine is 9064, and being a Schedule III substance it does not have an annual manufacturing quota imposed by the DEA.[44] The salt in use is the hydrochloride, which has a free base conversion ratio of 0.928.
In the years prior to Suboxone's approval, Reckitt Benckiser had lobbied Congress to help craft the Drug Addiction Treatment Act of 2000 (DATA 2000), which gave authority to the Secretary of Health and Human Services to grant a waiver to physicians with certain training to prescribe and administer Schedule III, IV, or V narcotic drugs for the treatment of addiction or detoxification. Prior to the passage of this law, such treatment was not permitted in outpatient settings except for clinics designed specifically for drug addiction.[45]
The waiver, which can be granted after the completion of an eight-hour course, is required for outpatient treatment of opioid addiction with Subutex and Suboxone. Initially, the number of patients each approved physician could treat was limited to ten. This was eventually modified to allow approved physicians to treat up to a hundred patients with buprenorphine for opioid addiction in an outpatient setting.[46] This limit was recently increased by the Obama administration, raising the number of patients to which doctors can prescribe to 275.[47] Still, due to this patient limit and the requisite eight-hour training course, many continuing patients can find it very difficult to get a prescription, despite the drug's effectiveness.[48]
In the European Union, Subutex and Suboxone, buprenorphine's high-dose sublingual tablet preparations, were approved for opioid addiction treatment in September 2006.[49] In the Netherlands, buprenorphine is a List II drug of the Opium Law, though special rules and guidelines apply to its prescription and dispensation.
Brand names
Buprenorphine is available under the trade names Cizdol, Suboxone, Subutex (typically used for opioid addiction), Temgesic (sublingual tablets for moderate to severe pain), Buprenex (solutions for injection often used for acute pain in primary-care settings), Norspan and Butrans (transdermal preparations used for chronic pain).[7]
Buprenorphine has been introduced in most European countries as a transdermal formulation (marketed as Transtec) for the treatment of chronic pain not responding to non-opioids.
Research
Depression
A clinical trial conducted at Harvard Medical School in the mid-1990s demonstrated that a majority of persons with non‑psychotic unipolar depression who were refractory to conventional antidepressants and electroconvulsive therapy could be successfully treated with buprenorphine.[50][51][52][53][54][55][56] Clinical depression is currently not an approved indication for the use of any opioid.
Buprenorphine/samidorphan (ALKS-5461), a combination product of buprenorphine and samidorphan (a preferential μ-opioid receptor antagonist), is currently undergoing phase III clinical trials in the United States for augmentation of antidepressant therapy for treatment-resistant depression.[57]
Cocaine dependence
In combination with samidorphan or naltrexone (μ-opioid receptor antagonists), buprenorphine is under investigation for the treatment of cocaine dependence, and recently demonstrated effectiveness for this indication in a large-scale (n = 302) clinical trial (at a high buprenorphine dose of 16 mg but not a low dose of 4 mg).[58][59]
Neonatal abstinence
Buprenorphine has been used in the treatment of the neonatal abstinence syndrome,[60] a condition in which newborns exposed to opioids during pregnancy demonstrate signs of withdrawal.[61] Use currently is limited to infants enrolled in a clinical trial conducted under an FDA approved investigational new drug (IND) application.[62] An ethanolic formulation used in neonates is stable at room temperature for at least 30 days.[63]
Obsessive-compulsive disorder
In one study, buprenorphine was found to be effective in a subset of individuals with treatment-refractory obsessive-compulsive disorder.[64]
References
- ↑ Mendelson J, Upton RA, Everhart ET, Jacob P 3rd, Jones RT (1997). "Bioavailability of sublingual buprenorphine.". Journal of Clinical Pharmacology. 37 (1): 31–7. PMID 9048270. doi:10.1177/009127009703700106.
- ↑ Eriksen J, Jensen NH, Kamp-Jensen M, Bjarnø H, Friis P, Brewster D (1989). "The systemic availability of buprenorphine administered by nasal spray". J. Pharm. Pharmacol. 41 (11): 803–5. PMID 2576057. doi:10.1111/j.2042-7158.1989.tb06374.x.
- 1 2 Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ↑ "Buprenorphine Hydrochloride". drugs.com. American Society of Health-System Pharmacists. 26 January 2017. Retrieved 17 March 2017.
- ↑ Claude, Andrew (June 2015). "Buprenorphine" (PDF). cliniciansbrief.com. Retrieved 25 February 2017.
- ↑ Kukanich, Butch; Papich, Mark G. (May 14, 2013). "Opioid Analgesic Drugs". In Jim E. Riviere, Mark G. Papich. Veterinary Pharmacology and Therapeutics (9 ed.). John Wiley & Sons. pp. 323–325. ISBN 9781118685907.
- 1 2 3 4 "Buprenorphine". Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. 14 January 2014. Retrieved 6 April 2014.
- ↑ Mattick, Richard P.; Breen, Courtney; Kimber, Jo; Davoli, Marina (2014-02-06). "Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence". The Cochrane Database of Systematic Reviews (2): CD002207. ISSN 1469-493X. PMID 24500948. doi:10.1002/14651858.CD002207.pub4.
- ↑ Glickman L, Galanter M, Dermatis H, Dingle S (December 2006). "Recovery and spiritual transformation among peer leaders of a modified methadone anonymous group". J Psychoactive Drugs. 38 (4): 531–3. PMID 17373569. doi:10.1080/02791072.2006.10400592.
Gilman SM, Galanter M, Dermatis H (December 2001). "Methadone Anonymous: A 12-Step Program for Methadone Maintained Heroin Addicts". Subst Abus. 22 (4): 247–256. PMID 12466684. doi:10.1080/08897070109511466.
McGonagle D (October 1994). "Methadone anonymous: a 12-step program. Reducing the stigma of methadone use". J Psychosoc Nurs Ment Health Serv. 32 (10): 5–12. PMID 7844771. - ↑ Horvath AT (2000). "Smart Recovery®: Addiction Recovery Support from a Cognitive-Behavioral Perspective". Journal of Rational-Emotive and Cognitive-Behavior Therapy. 18 (3): 181–191. doi:10.1023/A:1007831005098.
- ↑ "Butrans Medication Guide". Butrans Medication Guide. Purdue Pharma L.P. Retrieved 7 July 2014.
- ↑ Nutt, D; King, LA; Saulsbury, W; Blakemore, C (24 March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse.". Lancet (London, England). 369 (9566): 1047–53. PMID 17382831. doi:10.1016/S0140-6736(07)60464-4.
- 1 2 Budd K, Raffa RB. (eds.) Buprenorphine – The unique opioid analgesic. Thieme, 200, ISBN 3-13-134211-0
- ↑ "About Buprenorphine Therapy". Substance Abuse and Mental Health Services Administration. Archived from the original on 15 December 2014. Retrieved 15 December 2014.
- ↑ van Dorp E, Yassen A, Sarton E, Romberg R, Olofsen E, Teppema L, Danhof M, Dahan A (2006). "Naloxone reversal of buprenorphine-induced respiratory depression". Anesthesiology. 105 (1): 51–7. PMID 16809994. doi:10.1097/00000542-200607000-00012.
- ↑ Alford, Daniel P.; Compton, Peggy; Samet, Jeffrey H. (17 January 2006). "Acute Pain Management for Patients Receiving Maintenance Methadone or Buprenorphine Therapy". Annals of Internal Medicine. 144 (2): 127–134. ISSN 0003-4819. PMC 1892816
 . PMID 16418412. doi:10.7326/0003-4819-144-2-200601170-00010.
. PMID 16418412. doi:10.7326/0003-4819-144-2-200601170-00010. - ↑ Khroyan TV, Wu J, Polgar WE, et al. (June 2014). "BU08073 a Buprenorphine Analog with Partial Agonist Activity at mu Receptors in vitro but Long-Lasting Opioid Antagonist Activity in vivo in Mice". Br. J. Pharmacol. 172 (2): 668–680. PMC 4292977
 . PMID 24903063. doi:10.1111/bph.12796.
. PMID 24903063. doi:10.1111/bph.12796. - ↑ Jacob JJ, Michaud GM, Tremblay EC (1979). "Mixed agonist-antagonist opiates and physical dependence". Br J Clin Pharmacol. 7 Suppl 3: 291S–296S. PMC 1429306
 . PMID 572694. doi:10.1111/j.1365-2125.1979.tb04703.x.
. PMID 572694. doi:10.1111/j.1365-2125.1979.tb04703.x. - ↑ Lutfy K, Cowan A (October 2004). "Buprenorphine: a unique drug with complex pharmacology". Curr Neuropharmacol. 2 (4): 395–402. PMC 2581407
 . PMID 18997874. doi:10.2174/1570159043359477.
. PMID 18997874. doi:10.2174/1570159043359477. - ↑ Kress HG (March 2009). "Clinical update on the pharmacology, efficacy and safety of transdermal buprenorphine". Eur J Pain. 13 (3): 219–30. PMID 18567516. doi:10.1016/j.ejpain.2008.04.011.
- ↑ Robinson SE (2002). "Buprenorphine: an analgesic with an expanding role in the treatment of opioid addiction". CNS Drug Rev. 8 (4): 377–90. PMID 12481193.
- ↑ Pedro Ruiz; Eric C. Strain (2011). Lowinson and Ruiz's Substance Abuse: A Comprehensive Textbook. Lippincott Williams & Wilkins. p. 439. ISBN 978-1-60547-277-5.
- ↑ Bidlack JM (2014). "Mixed kappa/mu partial opioid agonists as potential treatments for cocaine dependence". Adv. Pharmacol. Advances in Pharmacology. 69: 387–418. ISBN 9780124201187. PMID 24484983. doi:10.1016/B978-0-12-420118-7.00010-X.
- ↑ Ehrich, Elliot; Turncliff, Ryan; Du, Yangchun; Leigh-Pemberton, Richard; Fernandez, Emilio; Jones, Reese; Fava, Maurizio (2014). "Evaluation of Opioid Modulation in Major Depressive Disorder". Neuropsychopharmacology. 40 (6): 1448–55. ISSN 0893-133X. PMC 4397403
 . PMID 25518754. doi:10.1038/npp.2014.330.
. PMID 25518754. doi:10.1038/npp.2014.330. - ↑ Mizoguchi H, Wu HE, Narita M, et al. (2002). "Antagonistic property of buprenorphine for putative epsilon-opioid receptor-mediated G-protein activation by beta-endorphin in pons/medulla of the mu-opioid receptor knockout mouse". Neuroscience. 115 (3): 715–21. PMID 12435410. doi:10.1016/s0306-4522(02)00486-4.
- ↑ Mizoguchi H, Spaulding A, Leitermann R, Wu HE, Nagase H, Tseng LF (July 2003). "Buprenorphine blocks epsilon- and micro-opioid receptor-mediated antinociception in the mouse". J. Pharmacol. Exp. Ther. 306 (1): 394–400. PMID 12721333. doi:10.1124/jpet.103.048835.
- ↑ Harold E. Doweiko (14 March 2014). Concepts of Chemical Dependency. Cengage Learning. pp. 149–. ISBN 978-1-285-45717-8.
- ↑ USP DI. United States Pharmacopeial Convention.
- ↑ Leffler A, Frank G, Kistner K, et al. (June 2012). "Local anesthetic-like inhibition of voltage-gated Na(+) channels by the partial μ-opioid receptor agonist buprenorphine". Anesthesiology. 116 (6): 1335–46. PMID 22504149. doi:10.1097/ALN.0b013e3182557917.
- ↑ Hutchinson, Mark R.; Zhang, Yingning; Shridhar, Mitesh; Evans, John H.; Buchanan, Madison M.; Zhao, Tina X.; Slivka, Peter F.; Coats, Benjamen D.; Rezvani, Niloofar; Wieseler, Julie; Hughes, Travis S.; Landgraf, Kyle E.; Chan, Stefanie; Fong, Stephanie; Phipps, Simon; Falke, Joseph J.; Leinwand, Leslie A.; Maier, Steven F.; Yin, Hang; Rice, Kenner C.; Watkins, Linda R. (2010). "Evidence that opioids may have toll-like receptor 4 and MD-2 effects". Brain, Behavior, and Immunity. 24 (1): 83–95. ISSN 0889-1591. PMC 2788078
 . PMID 19679181. doi:10.1016/j.bbi.2009.08.004.
. PMID 19679181. doi:10.1016/j.bbi.2009.08.004. - ↑ Xu J, Xu M, Hurd YL, Pasternak GW, Pan YX (2009). "Isolation and characterization of new exon 11-associated N-terminal splice variants of the human mu opioid receptor gene". J. Neurochem. 108 (4): 962–72. PMC 2727151
 . PMID 19077058. doi:10.1111/j.1471-4159.2008.05833.x.
. PMID 19077058. doi:10.1111/j.1471-4159.2008.05833.x. - ↑ Grinnell S et al (2014): Buprenorphine analgesia requires exon 11-associated mu opioid receptor splice variants. The FASEB Journal
- ↑ Moody DE, Fang WB, Lin SN, Weyant DM, Strom SC, Omiecinski CJ (2009). "Effect of Rifampin and Nelfinavir on the Metabolism of Methadone and Buprenorphine in Primary Cultures of Human Hepatocytes". Drug Metabolism and Disposition. 37 (12): 2323–2329. PMC 2784702
 . PMID 19773542. doi:10.1124/dmd.109.028605.
. PMID 19773542. doi:10.1124/dmd.109.028605. - ↑ Yassen A, Kan J, Olofsen E, Suidgeest E, Dahan A, Danhof M (2007). "Pharmacokinetic-pharmacodynamic modeling of the respiratory depressant effect of norbuprenorphine in rats". The Journal of Pharmacology and Experimental Therapeutics. 321 (2): 598–607. PMID 17283225. doi:10.1124/jpet.106.115972.
- ↑ Huang P, Kehner GB, Cowan A, Liu-Chen LY (2001). "Comparison of pharmacological activities of buprenorphine and norbuprenorphine: Norbuprenorphine is a potent opioid agonist". The Journal of Pharmacology and Experimental Therapeutics. 297 (2): 688–695. PMID 11303059.
- 1 2 3 Brown SM, Campbell SD, Crafford A, Regina KJ, Holtzman MJ, Kharasch ED (October 2012). "P-glycoprotein is a major determinant of norbuprenorphine brain exposure and antinociception". J. Pharmacol. Exp. Ther. 343 (1): 53–61. PMC 3464040
 . PMID 22739506. doi:10.1124/jpet.112.193433.
. PMID 22739506. doi:10.1124/jpet.112.193433. - ↑ Brown SM, Holtzman M, Kim T, Kharasch ED (2011). "Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active". Anesthesiology. 115 (6): 1251–60. PMC 3560935
 . PMID 22037640. doi:10.1097/ALN.0b013e318238fea0.
. PMID 22037640. doi:10.1097/ALN.0b013e318238fea0. - ↑ Heel RC, Brogden RN, Speight TM, Avery GS (February 1979). "Buprenorphine: a review of its pharmacological properties and therapeutic efficacy.". Drugs. 17 (2): 81–110. PMID 378645. doi:10.2165/00003495-197917020-00001.
- ↑ Baselt, R. (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. pp. 190–192. ISBN 0962652377.
- ↑ "IMPORTANT SAFETY INFORMATION".
- ↑ Campbell N. D.; Lovell A. M. (2012). "The history of the development of buprenorphine as an addiction therapeutic". Annals of the New York Academy of Sciences. 1248: 124–139. Bibcode:2012NYASA1248..124C. PMID 22256949. doi:10.1111/j.1749-6632.2011.06352.x.
- ↑ Louis S. Harris, ed. (1998). Problems of Drug Dependence, 1998: Proceedings of the 66th Annual Scientific Meeting, The College on Problems of Drug Dependence, Inc. (PDF). NIDA Research Monograph 179.
- ↑ Subutex and Suboxone Approval Letter. U.S. Food and Drug Administration (October 8, 2002). fda.gov.
- ↑ Quotas – Conversion Factors for Controlled Substances. Deadiversion.usdoj.gov. Retrieved on 2016-11-07.
- ↑ "Drug Addiction Treatment Act of 2000" Archived 2013-03-04 at the Wayback Machine.. SAMHSA, U.S. Department of Health & Human Services.
- ↑ The National Alliance of Advocates for Buprenorphine Treatment. naabt.org. Retrieved on 2013-05-19.
- ↑ Obama administration's change on buprenorphine policy. Business Insider (2016-07-06). Retrieved on 2016-11-07.
- ↑ Practically a book review: Dying to be Free. Slate Star Codex. Retrieved June 2015
- ↑ Suboxone EU Approval. Ema.europa.eu. Retrieved on 2016-11-07.
- ↑ Bodkin JA, Zornberg GL, Lukas SE, Cole JO (1995). "Buprenorphine treatment of refractory depression". Journal of Clinical Psychopharmacology. 15 (1): 49–57. PMID 7714228. doi:10.1097/00004714-199502000-00008.
- ↑ Emrich HM, Vogt P, Herz A (1982). "Possible antidepressive effects of opioids: Action of buprenorphine". Annals of the New York Academy of Sciences. 398: 108–112. Bibcode:1982NYASA.398..108E. PMID 6760767. doi:10.1111/j.1749-6632.1982.tb39483.x.
- ↑ Emrich HM (1984). "Endorphins in psychiatry". Psychiatr Dev. 2 (2): 97–114. PMID 6091098.
- ↑ Mongan L, Callaway E (1990). "Buprenorphine responders". BiolPsychiatry. 28 (12): 1078–1080. PMID 2289007. doi:10.1016/0006-3223(90)90619-d.
- ↑ Nyhuis PW, Gastpar M (2005). "Opiate treatment in ECT-resistant depression". Pharmacopsychiatry. 38 (5). doi:10.1055/s-2005-918797.
- ↑ Nyhuis PW, Specka M, Gastpar M (2006). "Does the antidepressive response to opiate treatment describe a subtype of depression?". European Neuropsychopharmacology. 16 (S16): S309. doi:10.1016/S0924-977X(06)70328-5. Retrieved 21 September 2012.
- ↑ Nyhuis PW, Gastpar M, Scherbaum N (2008). "Opiate Treatment in Depression Refractory to Antidepressants and Electroconvulsive Therapy". Journal of Clinical Psychopharmacology. 28 (5): 593–595. PMID 18794671. doi:10.1097/JCP.0b013e31818638a4.
- ↑ Alkermes (2014). "Alkermes Announces Initiation of FORWARD-3 and FORWARD-4 Efficacy Studies in Pivotal Program for ALKS 5461 for Treatment of Major Depressive Disorder".
- ↑ Ling, Walter; Hillhouse, Maureen P.; Saxon, Andrew J.; Mooney, Larissa J.; Thomas, Christie M.; Ang, Alfonso; Matthews, Abigail G.; Hasson, Albert; Annon, Jeffrey; Sparenborg, Steve; Liu, David S.; McCormack, Jennifer; Church, Sarah; Swafford, William; Drexler, Karen; Schuman, Carolyn; Ross, Stephen; Wiest, Katharina; Korthuis, Philip; Lawson, William; Brigham, Gregory S.; Knox, Patricia C.; Dawes, Michael; Rotrosen, John (2016). "Buprenorphine + Naloxone plus Naltrexone for the Treatment of Cocaine Dependence:The Cocaine Use Reduction with Buprenorphine(CURB)Study". Addiction. 111 (8): 1416–1427. ISSN 0965-2140. PMC 4940267
 . PMID 26948856. doi:10.1111/add.13375.
. PMID 26948856. doi:10.1111/add.13375. - ↑ Reuters (2012). "Alkermes Presents Positive Clinical Data of ALKS 5461 at 52nd Annual New Clinical Drug Evaluation Unit Meeting".
- ↑ Kraft WK, Gibson E, Dysart K, Damle VS, Larusso JL, Greenspan JS, Moody DE, Kaltenbach K, Ehrlich ME (September 2008). "Sublingual buprenorphine for treatment of neonatal abstinence syndrome: a randomized trial". Pediatrics. 122 (3): e601–7. PMC 2574639
 . PMID 18694901. doi:10.1542/peds.2008-0571.
. PMID 18694901. doi:10.1542/peds.2008-0571. - ↑ Kraft WK, van den Anker JN (2012). "Pharmacologic Management of the Opioid Neonatal Abstinence Syndrome". Pediatric Clinics of North America. 59 (5): 1147–1165. PMC 4709246
 . PMID 23036249. doi:10.1016/j.pcl.2012.07.006.
. PMID 23036249. doi:10.1016/j.pcl.2012.07.006. - ↑ Buprenorphine for the Treatment of Neonatal Abstinence Syndrome. Clinicaltrials.gov. NCT00521248. Retrieved on 2013-05-19.
- ↑ Anagnostis EA, Sadaka RE, Sailor LA, Moody DE, Dysart KC, Kraft WK (2011). "Formulation of buprenorphine for sublingual use in neonates". The journal of pediatric pharmacology and therapeutics. 16 (4): 281–284. PMC 3385042
 . PMID 22768012.
. PMID 22768012. - ↑ Liddell, M. B.; Aziz, V.; Briggs, P.; Kanakkehewa, N.; Rawi, O. (2012). "Buprenorphine augmentation in the treatment of refractory obsessive-compulsive disorder". Therapeutic Advances in Psychopharmacology. 3 (1): 15–19. ISSN 2045-1253. PMC 3736962
 . PMID 23983988. doi:10.1177/2045125312462233.
. PMID 23983988. doi:10.1177/2045125312462233.
External links
- U.S. Federal government buprenorphine program for opioid addiction
- Australian national buprenorphine policy
- The bitter pill: A Wired Magazine article on Suboxone
- Subu Must Die – How a nation of junkies went cold turkey: A New Republic article on Subutex abuse in the nation of Georgia