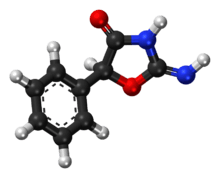
Pemoline
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cylert |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 50% bound to plasma proteins |
| Metabolism | Hepatic |
| Biological half-life | 12 hours |
| Excretion | ? |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.016.763 |
| Chemical and physical data | |
| Formula | C9H8N2O2 |
| Molar mass | 176.172 g/mol |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Pemoline is a stimulant drug of the 4-oxazolidinone class. It was first synthesized in 1913[1] but its activity was not discovered until the 1930s.[2] Under the names Betanamin, Cylert, Tradon, and Ceractiv it was used as a medication to treat attention-deficit hyperactivity disorder (ADHD) and narcolepsy. Under the Convention on Psychotropic Substances, it is a Schedule IV drug.[3] It is no longer generally available in the United States as a result of the FDA withdrawing approval of pemoline as an indicated treatment for ADHD, due to its implication in liver failures among children who were receiving the medication. An FDA Alert warned against prescribing pemoline for ADHD. This spurred Abbott Laboratories, the patent owner of Cylert, to cease manufacturing Cylert. Manufacturers of the generic equivalents followed suit.
Mechanism of action
Pemoline is generally considered dopaminergic, but its precise method of action hasn't yet been definitively determined.[4] Pemoline passes the blood–brain barrier and acts as a surrogate for dopamine, not affecting endogenous intracellular dopamine. For this reason, and the fact that it has little or no affinity for adrenaline receptors, pemoline has minimal sympathomimetic side effects such as: dry mouth, reduction in appetite, high blood pressure, increased heart rate, constriction of smooth muscle, cardiac stress, dilated pupils and insomnia. There is some data to suggest that pemoline is a nootropic acting as a catalyst conductor in the synapses of the brain's memory centers, raising the efficiency of memory and assisting RNA formation in the brain. While drugs like dexamphetamine and methylphenidate are classified as Schedule II, pemoline is listed as Schedule IV (non-narcotic). In studies conducted on primates, pemoline fails to demonstrate a potential for self-administration.
Pemoline is Schedule IV Non-Narcotic (Stimulant) controlled substance with a DEA ACSCN of 1530 and is not subject to annual manufacturing quotas. The salts in use are pemoline magnesium (free base conversion ratio .751), pemoline iron (.578), pemoline copper (.644), pemoline nickel (.578), pemoline rubidium, pemoline calcium, pemoline chromium, and chelates of the above which are identical in weight to the salt mentioned. Pemoline free base and pemoline cobalt, strontium, silver, barium, lithium, sodium, potassium, zinc, manganese, and caesium are research chemicals which can be produced in situ for experiments.[5][6][7] Others such as lanthanide pemoline salts such as pemoline cerium can be prepared; pemoline beryllium would presumably be toxic.
Interactions
Other stimulants and MAOI’s are contraindicated with pemoline.
Liver toxicity
In some patients pemoline is suspected of causing hepatotoxicity,[8] so the FDA recommended that regular liver tests should be performed on those treated with it.[9] Since receiving FDA approval in 1975,[10] it has been linked with 21 cases of liver failure, of which 13 resulted in liver replacement or death.
In March 2005 Abbott Laboratories and generic manufacturers withdrew pemoline from the American market due to concerns about the liver toxicity risk.[11]
Overdose
Overdose of pemoline may present with choreoathetosis symptoms.[12]
Synthesis
Pemoline is synthesized by the condensation of racemic ethyl mandelate with guanidine.
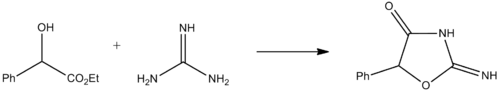
See also
Notes
- ↑ Chemische Berichte,1913,vol.46, p. 2083
- ↑ Acta Academiae Aboensis, Series B: Mathematica et Physica, 1939, vol. 11, #14 p. 3,7
- ↑ Annual Estimates Of Requirements Of Narcotic Drugs, Manufacture Of Synthetic Drugs, Opium Production And Cultivation Of The Archived December 5, 2005, at the Wayback Machine.
- ↑ "Cylert (Pemoline)" (PDF). FDA. December 2002. Retrieved 15 February 2014.
- ↑ DEA office of Diversion Control site: Federal Register publications of CSA schedules, 2014 Q1
- ↑ The A-Z Encyclopaedia of Alcohol and Drug Abuse
- ↑ CRC Handbook of Chemistry & Physics
- ↑ Marotta PJ, Roberts EA (May 1998). "Pemoline hepatotoxicity in children". J. Pediatr. 132 (5): 894–7. PMID 9602211. doi:10.1016/S0022-3476(98)70329-4.
- ↑ Willy ME, Manda B, Shatin D, Drinkard CR, Graham DJ (July 2002). "A study of compliance with FDA recommendations for pemoline (Cylert)". J Am Acad Child Adolesc Psychiatry. 41 (7): 785–90. PMID 12108802. doi:10.1097/00004583-200207000-00009.
- ↑ Etwel FA, Rieder MJ, Bend JR, Koren G (2008). "A surveillance method for the early identification of idiosyncratic adverse drug reactions". Drug Saf. 31 (2): 169–80. PMID 18217792. doi:10.2165/00002018-200831020-00006.
- ↑ "Pemoline - Withdrawn due to liver toxicity risk". WHO Pharmaceuticals Newsletter (05). 2005.
- ↑ Stork CM, Cantor R (1997). "Pemoline induced acute choreoathetosis: case report and review of the literature". J. Toxicol. Clin. Toxicol. 35 (1): 105–8. PMID 9022662. doi:10.3109/15563659709001175.
Additional references
- Budavari, S (Ed.) (1996). The Merck Index. An encyclopedia of chemicals, drugs, and biologicals (12 ed.). Whitehouse Station: Merck Research Laboratories ISBN 0-911910-12-3
- Michanie, Claudio: Diferencias del Trastorno por Déficit de Atención en el niño y el adulto: consideraciones diagnósticas y terapéuticas. En Julio Moizeszowicz (Ed.): Psicofarmacología Psicodinámica IV - Actualizaciones 2004 ISBN 987-43-8089-6
- Moizeszowicz, Julio: Psicofarmacología psicodinámica IV, Editorial Paidós, Buenos Aires (2000) ISBN 950-12-3180-1
- Wilens T et al.: The Stimulants. Psychiatric Clinics of North America, 1992; 15: 191-222.
External links
- Pemoline monograph at HealthyPlace.com
- AScribe Newswire press release about the withdrawal.
- Cylert Discontinued Press Release