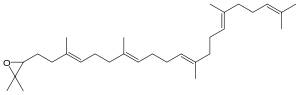
2,3-Oxidosqualene
 | |
| Names | |
|---|---|
| IUPAC name
2,2-Dimethyl-3-[(3E,7E,11E,15E)- 3,7,12,16,20-pentamethylhenicosa- 3,7,11,15,19-pentaenyl]oxirane | |
| Other names
Squalene oxide 2,3-Squalene oxide Squalene epoxide | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| MeSH | 2,3-oxidosqualene |
| PubChem CID |
|
| |
| |
| Properties | |
| C30H50O | |
| Molar mass | 426.717 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
(S)-2,3-Oxidosqualene ((S)-2,3-epoxysqualene) is an intermediate in the synthesis of the cell membrane sterol precursors lanosterol and cycloartenol, as well as saponins. It is formed when squalene is oxidized by the enzyme squalene monooxygenase. 2,3-Oxidosqualene is the substrate of various oxidosqualene cyclases, including lanosterol synthase, which produces lanosterol, a precursor to cholesterol.[1]
The stereoisomer 2,3-(R)-oxidosqualene is an inhibitor of lanosterol synthase.
References
- ↑ Abe I. (2007). "Enzymatic synthesis of cyclic triterpenes". Natural Products Reports. 24 (6): 1311–31. PMID 18033581. doi:10.1039/b616857b.
External links
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.