Spirolactone
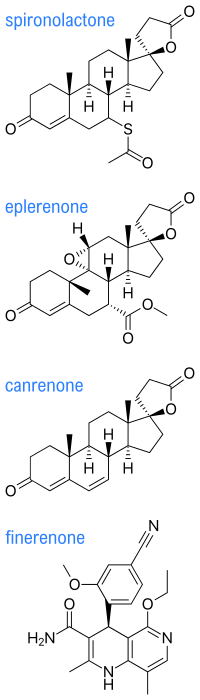
Spirolactones, also known as steroid-17α-spirolactones, 17α-spirolactosteroids, or simply 17α-spirolactones, are a group of steroids which are both spiro compounds and lactones and which feature these two structural properties combined in the form of a spirolactone ring at the C17α position.[1][2][3] They are antimineralocorticoids, or antagonists of the mineralocorticoid receptor (which is activated predominantly by the mineralocorticoid steroid hormone aldosterone), and have been employed clinically as potassium-sparing diuretics.[1][3][4][5] Some also possess progestogenic and/or antiandrogen properties, which have both contributed to side effects and been utilized for medical indications (e.g., spironolactone as an antiandrogen, and drospirenone as a progestin).[1][3][6] The spirolactones were developed by G. D. Searle & Company in the 1950s and thereafter and were denoted as "SC" compounds (e.g., SC-9420 for spironolactone).[1][5]
The spirolactones include the marketed drugs spironolactone (SC-9420; Aldactone), canrenone (SC-9376; Cantaren, Luvion), potassium canrenoate (SC-14266; Venactone, Soldactone), eplerenone (SC-66110, CGP-30083; Inspra), and drospirenone (ZK-30595; Yasmin). Spirolactones that were not ever marketed include SC-5233,[4] SC-8109,[4] SC-11927 (Catatoxic Steroid 1; CS-1), spiroxasone, prorenone (SC-23133), prorenoate potassium (SC-23992), 7α-thiospironolactone (SC-24813), mexrenone (SC-25152, ZK-32055), dicirenone (SC-26304), 7α-thiomethylspironolactone (SC-26519), mexrenoate potassium (SC-26714), spirorenone (ZK-35973), ZK-91587 (15β,16β-methylenemexrenone), mespirenone (ZK-94679), and ZK-97894 (7α-thiomethylmespirenone). Oxprenoate potassium (RU-28318) is not a spirolactone by definition but is a closely related antimineralocorticoid that was never marketed.
SC-5233 (6,7-dihydrocanrenone), the C17α propanoic acid lactone of testosterone (androst-4-en-17β-ol-3-one), is the unsubstituted parent or prototype compound of the spirolactones, and is one of a few of the simplest members of the series along with SC-8109 (the 19-demethyl analogue of SC-5233) and canrenone (the 1,2-didehydro analogue of SC-5233).[1][2][7] Spironolactone is a derivative of SC-5233 with a 7α-acetylthio group (that is, SC-5233 is 7α-desthioacetylspironolactone).[1]
See also
- 7α-Thioprogesterone (SC-8365)
References
- 1 2 3 4 5 6 P. J. Bentley (1980). Endocrine Pharmacology: Physiological Basis and Therapeutic Applications. CUP Archive. pp. 159–160. ISBN 978-0-521-22673-8.
- 1 2 Gyorgy Szasz; Zsuzsanna Budvari-Barany (19 December 1990). Pharmaceutical Chemistry of Antihypertensive Agents. CRC Press. pp. 82–. ISBN 978-0-8493-4724-5.
- 1 2 3 Luther, James M. (2014). "Is there a new dawn for selective mineralocorticoid receptor antagonism?". Current Opinion in Nephrology and Hypertension. 23 (5): 456–461. ISSN 1062-4821. doi:10.1097/MNH.0000000000000051.
- 1 2 3 E. Buchborn; K. D. Bock (14 December 2013). Diuresis and Diuretics / Diurese und Diuretica: An International Symposium Herrenchiemsee, June 17th–20th, 1959 Sponsored by CIBA / Ein Internationales Symposium Herrenchiemsee, 17.–20. Juni 1959 Veranstaltet mit Unterstützung der CIBA. Springer-Verlag. pp. 261–. ISBN 978-3-642-49716-2.
- 1 2 Rainer F. Greger; H. Knauf; E. Mutschler (6 December 2012). Diuretics. Springer Science & Business Media. pp. 335–. ISBN 978-3-642-79565-7.
- ↑ Risto Erkkola (2006). The Menopause. Elsevier. pp. 188–. ISBN 978-0-444-51830-9.
- ↑ Janos Fischer; C. Robin Ganellin (24 August 2010). Analogue-based Drug Discovery II. John Wiley & Sons. pp. 361–. ISBN 978-3-527-63212-1.