Saccharin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2H-1λ6,2-Benzothiazol-1,1,3-trione | |
| Other names
Benzoic sulfimide
| |
| Identifiers | |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.202 |
| E number | E954 (glazing agents, ...) |
| KEGG | |
| PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C7H5NO3S | |
| Molar mass | 183.18 g·mol−1 |
| Appearance | White crystalline solid |
| Density | 0.828 g/cm3 |
| Melting point | 228.8 to 229.7 °C (443.8 to 445.5 °F; 501.9 to 502.8 K) |
| 1 g per 290 mL | |
| Acidity (pKa) | 1.6[2] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Sodium Saccharin (benzoic sulfimide) is an artificial sweetener with effectively no food energy that is about 300–400 times as sweet as sucrose but has a bitter or metallic aftertaste, especially at high concentrations. It is used to sweeten products such as drinks, candies, cookies, and medicines.[3]
Etymology
Saccharin derives its name from the word "saccharine", meaning "sugary". The word saccharine is used figuratively, often in a derogative sense, to describe something "unpleasantly over-polite" or "overly sweet".[4] Both words are derived from the Greek word σάκχαρον (sakcharon) meaning "gravel".[5]
Properties

Saccharin is heat stable. It does not react chemically with other food ingredients; as such, it stores well. Blends of saccharin with other sweeteners are often used to compensate for each sweetener's weaknesses and faults. A 10:1 cyclamate:saccharin blend is common in countries where both these sweeteners are legal; in this blend, each sweetener masks the other's off taste. Saccharin is often used with aspartame in diet carbonated soft drinks, so some sweetness remains should the fountain syrup be stored beyond aspartame's relatively short shelf life.
In its acid form, saccharin is not water-soluble. The form used as an artificial sweetener is usually its sodium salt.[6] The calcium salt is also sometimes used, especially by people restricting their dietary sodium intake. Both salts are highly water-soluble: 0.67 g/ml water at room temperature.[7][8]
Safety and health effects
In the 1970s, studies performed on laboratory rats found an association between consumption of high doses of saccharin and the development of bladder cancer.[9] However, further study determined that this effect was due to a mechanism that is not relevant to humans.[9] Epidemiological studies have shown no evidence that saccharin is associated with bladder cancer in humans.[9][10] The International Agency for Research on Cancer (IARC) originally classified saccharin in Group 2B ("possibly carcinogenic to humans") based on the rat studies, but downgraded it to Group 3 ("not classifiable as to the carcinogenicity to humans") upon review of the subsequent research.[11]
Saccharin has no food energy and no nutritional value.[12] It is safe to consume for individuals with diabetes.[13][14]
History

Saccharin was produced first in 1879, by Constantin Fahlberg, a chemist working on coal tar derivatives in Ira Remsen's laboratory at the Johns Hopkins University.[15] Fahlberg noticed a sweet taste on his hand one evening, and connected this with the compound benzoic sulfimide on which he had been working that day.[16][17] Fahlberg and Remsen published articles on benzoic sulfimide in 1879 and 1880.[7][18] In 1884, then working on his own in New York City, Fahlberg applied for patents in several countries, describing methods of producing this substance that he named saccharin.[19] Two years later, he began production of the substance in a factory in a suburb of Magdeburg, Germany. Fahlberg would soon grow wealthy, while Remsen merely grew irritated, believing he deserved credit for substances produced in his laboratory. On the matter, Remsen commented, "Fahlberg is a scoundrel. It nauseates me to hear my name mentioned in the same breath with him."[20]
Although saccharin was commercialized not long after its discovery, until sugar shortages during World War I, its use had not become widespread. Its popularity further increased during the 1960s and 1970s among dieters, since saccharin is a calorie-free sweetener. In the United States, saccharin is often found in restaurants in pink packets; the most popular brand is "Sweet'n Low". Because of the difficulty of importing sugar from the West Indies The British Saccharin Company was founded in 1917 to produce Saccharin at its Paragon Works near Accrington, Lancashire. Production was licensed and controlled by the Board of Trade in London. Production continued on the site until 1926.
Government regulation
Starting in 1907, the United States Food and Drug Administration began investigating saccharin as a result of the Pure Food and Drug Act. Harvey Wiley, then the director of the bureau of chemistry for the FDA, viewed it as an illegal substitution of a valuable ingredient (sugar) by a less valuable ingredient. In a clash that had career consequences, Wiley told President Theodore Roosevelt, "Everyone who ate that sweet corn was deceived. He thought he was eating sugar, when in point of fact he was eating a coal tar product totally devoid of food value and extremely injurious to health." But Roosevelt himself was a consumer of saccharin, and, in a heated exchange, Roosevelt angrily answered Wiley by stating, "Anybody who says saccharin is injurious to health is an idiot." The episode proved the undoing of Wiley's career.[21]
In 1911, Food Inspection Decision 135 stated that foods containing saccharin were adulterated.[22] However, in 1912, Food Inspection Decision 142 stated that saccharin was not harmful.[23]
More controversy was stirred in 1969 with the discovery of files from the FDA's investigations of 1948 and 1949. These investigations, which had originally argued against saccharin use, were shown to prove little about saccharin being harmful to human health. In 1977, the FDA made an attempt to completely ban the substance,[8][24] following studies showing that the substance caused cancer in rats. The attempted ban was unsuccessful due to public opposition that was encouraged by industry advertisements,[24] and instead the following label was mandated: "Use of this product may be hazardous to your health. This product contains saccharin which has been determined to cause cancer in laboratory animals". That requirement was dropped in 2000 following new research that concluded humans reacted differently than rats and were not at risk of cancer at typical intake levels.[24] (See also: labeling below.) The sweetener has continued to be widely used in the United States and is now the third-most popular artificial sweetener behind sucralose and aspartame.
In the European Union, saccharin is also known by the E number (additive code) E954.
The current status of saccharin is that it is allowed in most countries, and countries such as Canada have lifted their previous ban of it as a food additive.[25] The claims that it is associated with bladder cancer were shown to be unfounded in experiments on primates.[26] (It is, however, prohibited to mail saccharin tablets or packets to France.)[27]
Saccharin was formerly on California's list of chemicals known to the state to cause cancer for the purposes of Proposition 65, but it was delisted in 2001.[28]
Warning label addition and removal
In 1958, the United States Congress amended the Food, Drugs, and Cosmetic Act of 1938 with the Delaney clause to mandate that the Food and Drug Administration not approve substances that "induce cancer in man, or, after tests, [are] found to induce cancer in animals." Studies in laboratory rats during the early 1970s linked saccharin with the development of bladder cancer in rodents. As a consequence, all food containing saccharin was labeled with a warning meeting the requirement of the Saccharin Study and Labeling Act of 1977.[29]
However, in 2000, the warning labels were removed because scientists learned that rodents, unlike humans, have a unique combination of high pH, high calcium phosphate, and high protein levels in their urine.[30][31] One or more of the proteins that are more prevalent in male rats combine with calcium phosphate and saccharin to produce microcrystals that damage the lining of the bladder. Over time, the rat's bladder responds to this damage by overproducing cells to repair the damage, which leads to tumor formation. Since this does not occur in humans, there is no elevated risk of bladder cancer.[32]
The delisting of saccharin led to legislation repealing the warning label requirement for products containing saccharin.[33] In 2001, the U.S. Food and Drug Administration and the state of California reversed their positions on saccharin, declaring it safe for consumption.[24] The FDA's decision followed a 2000 determination by the U.S. Department of Health and Human Services' National Toxicology Program to remove saccharin from its list of carcinogens.
The EPA has officially removed saccharin and its salts from their list of hazardous constituents and commercial chemical products. In a December 14, 2010 release, the EPA stated that saccharin is no longer considered a potential hazard to human health.[34]
Chemistry
Preparation
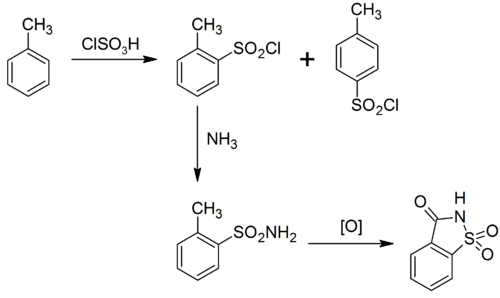
Saccharin can be produced in various ways.[35] The original route by Remsen and Fahlberg starts with toluene; another route begins with o-chlorotoluene.[36] Sulfonation of toluene by chlorosulfonic acid gives the ortho and para substituted sulfonyl chlorides. The ortho isomer is separated and converted to the sulfonamide with ammonia. Oxidation of the methyl substituent gives the carboxylic acid, which cyclicizes to give saccharin free acid:[37]
In 1950, an improved synthesis was developed at the Maumee Chemical Company of Toledo, Ohio. In this synthesis, the methyl anthranilate successively reacts with nitrous acid (from sodium nitrite and hydrochloric acid), sulfur dioxide, chlorine, and then ammonia to yield saccharin:[37]
Properties and reactions
The free acid of saccharin has a low pKa of 1.6 (the acidic hydrogen being that attached to the nitrogen).[2] Saccharin can be used to prepare exclusively disubstituted amines from alkyl halides via a nucleophilic substitution,[38] followed by Gabriel synthesis.[39][40]
See also
| Wikimedia Commons has media related to Saccharin. |
- Sugar substitute
- Assugrin (a brand containing saccharin and cyclamate)
- Cyclamate
- Sucralose
- Aspartame
- Neotame
- Steviol glycoside (natural sweetener)
- Xylitol (sugar alcohol; natural sweetener)
Notes and references
- ↑ Merck Index, 11th Edition, 8282.
- 1 2 Bell, R. P.; Higginson, W. C. E. (1949). "The Catalyzed Dehydration of Acetaldehyde Hydrate, and the Effect of Structure on the Velocity of Protolytic Reactions". Proc. R. Soc. A. 197 (1049): 141–159. doi:10.1098/rspa.1949.0055.
- ↑ "Saccharin (Inactive Ingredient)". drugs.com.
- ↑ "Saccharine". reference.com.
- ↑ "Saccharine". etymonline.com.
- ↑ Chattopadhyay, S; Raychaudhuri, U; Chakraborty, R (April 2014). "Artificial sweeteners - a review.". Journal of food science and technology. 51 (4): 611–21. PMC 3982014
 . PMID 24741154.
. PMID 24741154. - 1 2 Fahlberg, C. & Remsen, I. (1879). "Über die Oxydation des Orthotoluolsulfamids". Berichte der Deutschen chemischen Gesellschaft zu Berlin. 12: 469–473. doi:10.1002/cber.187901201135.
- 1 2 P. M. Priebem; G. B. Kauffman (1980). "Making governmental policy under conditions of scientific uncertainty: A century of controversy about saccharin in congress and the laboratory". Minerva. 18 (4): 556–574. PMID 11611011. doi:10.1007/BF01096124.
- 1 2 3 "Artificial Sweeteners and Cancer". National Cancer Institute.
- ↑ Weihrauch MR, Diehl V (2004). "Artificial sweeteners—do they bear a carcinogenic risk?". Ann Oncol. 15 (10): 1460–5. PMID 15367404. doi:10.1093/annonc/mdh256.
- ↑ "Saccharin: FDA Agencies". University of Minnesota, Environmental Health Sciences.
- ↑ "Common Terms: S-Z". American Diabetes Association.
- ↑ "Low-Calorie Sweeteners: What's News, What's New". American Diabetes Association.
- ↑ "Are Artificial Sweeteners Safe for People With Diabetes?". Cleveland Clinic.
- ↑ (As discussed below, the relative contributions of Fahlberg and Remsen to the discovery were later contested, with no final resolution in sight; the 1879 paper announcing the discovery lists both names as authors, with Fahlberg's name first.)
- ↑ Fahlberg's account of how he discovered the sweetness of saccharin appears in: (Anon.) (July 17, 1886) "The inventor of saccharine," Scientific American, new series, 60 (3) : 36.
- ↑ Myers, Rusty L.; Myers, Richard L. (2007). The 100 most important chemical compounds: a reference guide. Westport, Conn: Greenwood Press. p. 241. ISBN 0-313-33758-6.
- ↑ Remsen Ira; Fahlberg C. (February 1880). "On the oxidation of substitution products of aromatic hydrocarbons. IV. - On the oxidation of orthotoluenesulphamide". American Chemical Journal. 1 (6): 426–439. From pages 430-431: "It possesses a 'very marked sweet taste, being much sweeter than cane-sugar'. The taste is perfectly pure. The minutest quantity of the substance, a bit of its powder scarcely visible, if placed upon the tip of the tongue, causes a sensation of pleasant sweetness throughout the entire cavity of the mouth. As stated above, the substance is soluble to only a slight extent in cold water, but if a few drops of the cold aqueous solution be placed in an ordinary goblet full of water, the latter then tastes like the sweetest syrup. Its presence can hence easily be detected in the dilutest solutions by the taste."
- ↑ Constantin Fahlberg and Adolph List, "Manufacture of saccharine compounds," U.S. Patent no. 319,082 (filed: August 7, 1884).
- ↑ Getman, Frederick Hutton (1940). The life of Ira Remsen. Easton, Pa: Journal of chemical education. p. 66. OCLC 2640159
- ↑ "Sugar: A Cautionary Tale". www.fda.gov. Retrieved 2010-06-20.
- ↑ Charles Wesley Dunn (1913). Federal, State, and Territorial Reference Manual of Pure Food and Drug Law: Dunn's Pure Food and Drug Legal Manual. Dunn's pure food and drug legal manual corporation. pp. 1327–.
- ↑ California State Board of Health (1921). Monthly Bulletin. The Board. pp. 21–.
- 1 2 3 4 Conis, Elena. "Saccharin's mostly sweet following." Los Angeles Times. December 27, 2010, accessed January 14, 2011.
- ↑ , Health Canada
- ↑ Takayama, S.; Sieber, SM.; Adamson, RH.; Thorgeirsson, UP.; Dalgard, DW.; Arnold, LL.; Cano, M.; Eklund, S.; Cohen, SM. (Jan 1998). "Long-term feeding of sodium saccharin to nonhuman primates: implications for urinary tract cancer". J Natl Cancer Inst. 90 (1): 19–25. PMID 9428778. doi:10.1093/jnci/90.1.19.
- ↑ http://pe.usps.com/text/imm/fh_005.htm USPS Mailing Conditions
- ↑ Chemicals Delisted Effective April 6, 2001 as Known to the State to Cause Cancer, California Office of Environmental Health Hazard Assessment
- ↑ "Saccharin warning". AP via Telegraph-Herald. 1973-05-22. Retrieved 2011-06-09.
- ↑ Whysner, J.; Williams, GM. (1996). "Saccharin mechanistic data and risk assessment: urine composition, enhanced cell proliferation, and tumor promotion". Pharmacol Ther. 71 (1–2): 225–52. PMID 8910956. doi:10.1016/0163-7258(96)00069-1.
- ↑ Dybing, E. (Dec 2002). "Development and implementation of the IPCS conceptual framework for evaluating mode of action of chemical carcinogens". Toxicology. 181-182: 121–5. PMID 12505296. doi:10.1016/S0300-483X(02)00266-4.
- ↑ National Toxicology Program. Report on Carcinogens, Thirteenth Edition, Appendix B. pages 2-4. Linked from Saccharin index page at NTP, last updated on November 18, 2014. Page accessed Feb 29, 2016
- ↑ National Cancer Institute. Artificial Sweeteners and Cancer Last Reviewed: August 5, 2009. Page accessed Feb 29, 2016
- ↑ "EPA Removes Saccharin from Hazardous Substances Listing." December 14, 2010, accessed January 14, 2011.
- ↑ David J. Ager; David P. Pantaleone; Scott A. Henderson; Alan R. Katritzky; Indra Prakash; D. Eric Walters (1998). "Commercial, Synthetic Nonnutritive Sweeteners". Angewandte Chemie International Edition. 37 (13–24): 1802–23. doi:10.1002/(SICI)1521-3773(19980803)37:13/14<1802::AID-ANIE1802>3.0.CO;2-9.
- ↑ Bungard, G. (1967) Die SusStoffe Der Deut Apotheker, 19, 150.
- 1 2 Gert-Wolfhard von Rymon Lipinski (2005), "Sweeteners", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a26_023
- ↑ Ervithayasuporn, V.; Yingsukkamol, Pa-Kwan; Phurat, Chuttree; Somsook, Ekasith; Osotchan, Tanakorn; Ervithayasuporn, Vuthichai (2012). "Synthesis and Reactivity of Nitrogen Nucleophiles-Induced Cage-Rearrangement Silsesquioxanes". Inorg. Chem. 51 (22): 12266–12272. doi:10.1021/ic3015145.
- ↑ Ragnarsson, Ulf; Grehn, Leif (May 2002). "Novel Gabriel reagents". Accounts of Chemical Research. 24 (10): 285–289. doi:10.1021/ar00010a001.
- ↑ Sugasawa, S.; Abe, K. (1952). "A New Method for the Preparation of Secondary Amines. I Synthesis of Aliphatic Secondary Amines". Journal of the Pharmaceutical Society of Japan (Yakugaku Zasshi). 72 (2): 270–273. doi:10.1248/yakushi1947.72.2_270.