Sodium dodecylbenzenesulfonate
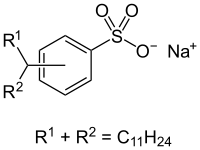 | |
| Names | |
|---|---|
| IUPAC name
sodium dodecylbenzenesulfonate | |
Other names
| |
| Identifiers | |
| Abbreviations | SDBS |
| ECHA InfoCard | 100.042.422 |
| PubChem CID |
|
| UNII |
|
| Properties | |
| C18H29NaO3S | |
| Molar mass | 348.48 g·mol−1 |
| 20% | |
| Hazards | |
| GHS pictograms |   [1] [1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Sodium dodecylbenzenesulfonates are organic compounds with the formula C12H25C6H4SO3Na. They are colourless salts with useful properties as surfactants. They are usually produced as a mixture of related sulfonates. They are major components of laundry detergent.[2]
Alkylbenzenesulfonates
Most sodium dodecylbenzenesulfonates are a member of the linear alkylbenzenesulfonates, meaning that the dodecyl group (C12H25) is unbranched. This dodecyl chain is attached at the 4-position of the benzenesulfonate group. Linear dodecyl-4-benzenesulfonate anions can exist in six isomers (ignoring optical isomers), depending on the carbon of the dodecyl group that is attached to the benzene ring. The isomer shown below left is 4-(5-dodecyl)benzenesulfonate (4 indicating the position of the benzene ring, 5 indicating the position on the dodecane chain). Branched isomers, e.g. those derived from tetramerized propylene, are also known (below right) but are not as widely used because they biodegrade too slowly.
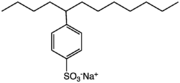 4-(5-Dodecyl) benzenesulfonate, a linear dodecylbenzenesulfonate
4-(5-Dodecyl) benzenesulfonate, a linear dodecylbenzenesulfonate A branched dodecylbenzenesulfonate, which has been phased out in developed countries.
A branched dodecylbenzenesulfonate, which has been phased out in developed countries.
Further complicating the description of the commercial materials, sodium dodecylbenzenesulfonate is one component of a mixture of compounds that feature variable alkyl chain lengths aside from C12, mainly ranging from C10-C16. Dodecylbenzenesulfonate is considered representative of the entire class of compounds, since the mean number of alkyl carbon atoms in the alkylbenzenesulfonates is 12.
Production
Billions of kilograms are produced annually. Alkylbenzenesulfonates have been prepared by many methods.[3] In the most common route, benzene is alkylated by long chain monoalkenes (e.g. dodecene) using hydrogen fluoride as a catalyst. The purified dodecylbenzenes (and related derivatives) are then sulfonated with sulfur trioxide to give the sulfonic acid. The sulfonic acid is subsequently neutralized with sodium hydroxide.[2]
Environmental considerations
Biodegradability has been well studied,[4][5] and is affected by the isomerization (branching). The salt of the linear material has an LD50 of 2.3 mg/liter for fish, about 4x more toxic than the branched compound; however the linear compound biodegrades far more quickly, making it the safer choice over time. It is biodegraded rapidly under aerobic conditions with a half-life of approximately 1–3 weeks;[4] oxidative degradation initiates at the alkyl chain.[2] Under anaerobic conditions it degrades very slowly or not at all, causing it to exist in high concentrations in sewage sludge, but this is not thought to be a cause for concern as it will rapidly degrade once returned to an oxygenated environment.
References
- ↑ "Dodecyl benzenesulfonic acid, sodium salt". GESTIS Substance Database.
- 1 2 3 Kurt Kosswig,"Surfactants" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, 2005, Weinheim. doi:10.1002/14356007.a25_747
- ↑ "5th World Conference on Detergents" Arno Cahn, ed., 2003. ISBN 1-893997-40-5.
- 1 2 Jensen, John (February 1999). "Fate and effects of linear alkylbenzene sulphonates (LAS) in the terrestrial environment". Science of The Total Environment. 226 (2-3): 93–111. PMID 10085562. doi:10.1016/S0048-9697(98)00395-7.
- ↑ Mackay, Donald; Di Guardo, Antonio; Paterson, Sally; Kicsi, Gabriel; Cowan, Christina E.; Kane, David M. (September 1996). "Assessment of chemical fate in the environment using evaluative, regional and local-scale models: Illustrative application to chlorobenzene and linear alkylbenzene sulfonates". Environmental Toxicology and Chemistry. 15 (9): 1638–1648. doi:10.1002/etc.5620150930.