Sic1
| Sic1 | |
|---|---|
| Identifiers | |
| Symbol | Sic1 |
| Alt. symbols | YLR079W, SDB25, SIC1_YEAST, CDK inhibitor p40 |
| Entrez | 850768 |
| UniProt | P38634 |
Sic1, a protein, is a stoichiometric inhibitor [1] of Cdk1-Clb (B-type cyclins) complexes in the budding yeast Saccharomyces cerevisiae. Because B-type cyclin-Cdk1 complexes are the drivers of S-phase initiation, Sic1 prevents premature S-phase entry.[2] Multisite phosphorylation of Sic1 is thought to time Sic1 ubiquitination and destruction, and by extension, the timing of S-phase entry.[3]
Role of Sic1 in the cell cycle control
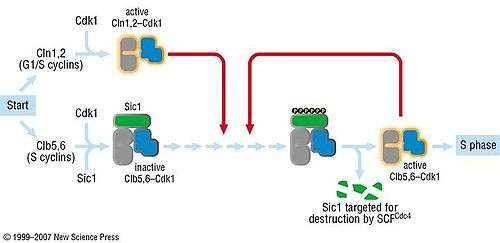
In the G1 phase of the cell cycle, Sic1 binds tightly to the Cdc28-Clb complex and inhibits it.[4] Low Cdc28-Clb activity leads to the disassembly of the mitotic spindle, the assembly of the prereplicative complex and initiation of bud formation in yeast.
At the START point in the yeast cell cycle, the G1-cyclins Cln3, Cln1 and Cln 2 activate Cdc28. The activated complex will phosphorylate Sic1 at multiple sites which leads to its degradation by the SCF complex.[5] When Sic1 is degraded, the Cdc28-Clb complex is no longer inhibited and the cell can enter the S/M-phase. Thus Sic1 inactivation is essential for transition into S phase (Fig.1).
Cdc28 in complex with B-type cyclin (Cdc28-Clb) phosphorylates Swi5, the transcription factor of Sic1. This promotes the export of Swi5 from the nucleus to the cytoplasm and avoids further transcription of the cdk inhibitor. Cdc28-Clb also phosphorylates any Sic1 molecules still available and triggers their ubiquitin-dependent degradation, exactly like Cdc28-Cln.[4] High Cdc28-Clb levels also initiate DNA replication and duplication of the spindle pole bodies (SPBs). Then the metaphase spindle assembles and chromosome segregation can occur. The transcription of Sic1 starts during telophase, mediated by Swi5. Aca2 is another transcription factor of Sic1, but remains inactive until G1.[6] At the end of mitosis, Sic1 is involved in the inactivation of Cdc28-Clb.[7]
Ubiquitin-dependent degradation of Sic1

In order to be recognized by Cdc4 of the SCF complex, Sic1 has to be phosphorylated, often by Cyclin-Cdk complexes, at least at 6 of the 9 cdk sites (Fig. 2).[8] Sic1 can also be phosphorylated by other kinases, such as Pho85-Pc11 , a kinase which becomes essential when Cln1 and Cln2 are absent.[9] Sic1 has also a role in the response to osmostress. The stress-activated protein kinase (SAPK) Hog1 phosphorylates Sic1 at a single residue at the carboxyl terminus. This leads to downregulation of cyclin expression and Sic1 stabilization which arrests the cell cycle.[10]
Multiple phosphorylations allow fine-tuning
Sic1 needs to be phosphorylated at multiple sites for ubiquitination-driven degradation (Fig. 2). The multiple phosphorylations are required for Sic1 to be recruited by Cdc4 to the SCF complex.[11] The Cdc4 substrate recognition mechanism includes the interaction with consensus binding motifs on the surface of the folded and phosphorylated Sic1, the so-called Cdc4 phospho-degrons (CPD). It has been shown that the optimal consensus sequence for Cdc4 is a phosphorylated serine or threonine followed by a proline and a basic amino acid. However, none of the CPDs on the surface of the Sic1 show such a composition. Therefore, multiple phosphorylation of Sic1 is necessary to get high-affinity binding to Cdc4.[8] Although this mechanism looks inefficient, it provides advantages for a cell because it is possible to measure the environmental Cln/cdc28 concentration. The number of phosphorylated sites corresponds to the concentration of Cln/cdc28 and Sic1 could be considered as a sensor for this protein. In contrast to the many sharp transitions of ultrasensitive kinase cascade feedback loops, this mechanism allows fine tuned regulation.[8] Moreover, because multiple phosphorylations are required, the probability that Sic1 is degraded by random is small. Using multiple phosphorylation of Sic1, the cell evolved a strategy to highly regulate the onset of DNA replication that is absolutely vital to provide genetic stability.
Sic1 homologue in human and diseases
The protein p27Kip1 is a human homologue of Sic1, both having a conserved inhibitory domain,[12] but p27Kip1 inhibits G1 cyclins and not cyclin B. There are several human diseases that are linked to p27Kip1 and other cyclin kinase inhibitors:
- All Papillary microcarcinomas (PMCs) of the thyroid have a lower expression of p27Kip1 than normal thyroid tissue. Additionally, the expression of p27Kip1 in more aggressive, metastasising Papillary microcarcinomas is strongly reduced compared to nonmetastasing microcarcinomas. These results suggest that p27Kip1 acts as a tumor suppressor.[13]
- Kaposi's sarcoma is a type of cancer which appears in combination with AIDS and presumably is caused by the human herpesvirus 8 (HHV8). This virus expresses a viral cyclin which builds a complex with Cdk6. This KSHV-cyclin-Cdk6 complex phosphorylates and destabilises p27Kip1, which results in a low level of p27Kip1. This suggests that the degradation of p27Kip1 is associated with the development of the tumors.[14]
- Patients with a gastric adenocarcinoma (stomach cancer) have a higher chance of survival if the tumor has high p27Kip1 expression. Low p27Kip1 expression can lead to tumor de-differentiation, increased penetration through the gastric wall, lymph node metastasis and advanced tumor stage.[15]
Thus, the human Cdk inhibitor p27Kip1 is a potential tumor suppressor protein. If its expression is reduced, the result might be unregulated progression from G1 to S-phase which deregulates cell division and simplifies the formation of tumors.
See also
References
- ↑ Schwob, E., T. Bohm, M. D. Mendenhall and K. Nasmyth (1994). "The B-type cyclin kinase inhibitor p40SIC1 controls the G1 to S transition in S. cerevisiae.". Cell. 79 (2): 233–244. PMID 7954792. doi:10.1016/0092-8674(94)90193-7.
- ↑ Morgan, David O., The Cell Cycle: Principles of Control, New Science Press, 1997. pg. 200-201.
- ↑ F. Tripodi; V. Zinzalla; M. Vanoni; L. Alberghina; P. Coccetti (2007). "In CK2 inactivated cells the cyclin dependent kinase inhibitor Sic1 is involved in cell-cycle arrest before the onset of S phase". Biochemical and Biophysical Research Communications. 359 (4): 921–927. PMID 17574209. doi:10.1016/j.bbrc.2007.05.195.
- 1 2 F.R. Cross; l. Schoeder; J.M. Bean (2007). "Phosphorylation of the Sic1 inhibitor of B-type cyclins in Saccharomyces cerevisiae is not essential but contributes to cell cycle robustness.". Genetics. 176 (3): 1541–1555. PMC 1931548
 . PMID 17483408. doi:10.1534/genetics.107.073494.
. PMID 17483408. doi:10.1534/genetics.107.073494. - ↑ R. Verma; R.S. Annan; M.J. Huddleston; S.A. Carr; G. Reynard; R.J. Deshaies (1997). "Phosphorylation of Sic1p by G1 Cdk required for its degradation and entry into S phase". Science. 278 (5337): 455–460. PMID 9334303. doi:10.1126/science.278.5337.455.
- ↑ J.H. Toyn; A.L. Johnson; J.D. Donocan; W.M. Toone; L.H. Johnston (1997). "The Swi5 transcription factor of Saccharomyces cerevisiae has a role in exit from mitosis through induction of the cdk-inhibitor Sic1 in telophase". Genetics. 145 (1): 85–96. PMC 1207787
 . PMID 9017392.
. PMID 9017392. - ↑ A. Calzada; M. Sacristan; E. Sanchez; A. Bueno (2001). "Cdc6 cooperates with Sic1 and Hct1 to inactivate mitotic cyclin-dependent kinases". Nature. 412 (6844): 355–358. PMID 11460169. doi:10.1038/35085610.
- 1 2 3 P. Nash, X. Tang, S. Orlicky, Q. Chen, F.B. Gertler, M.D. Mendenhall, F. Sicheri, T. Pawson, M. Tyers (2001). "Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication". Nature. 414 (6863): 514–21. PMID 11734846. doi:10.1038/35107009.
- ↑ M. Nishizawa; M. Kawasumi; M. Fujino; A. Toh-e (1998). "Phosphorylation of sic1, a cyclin-dependent kinase (Cdk) inhibitor, by Cdk including Pho85 kinase is required for its prompt degradation". Molecular Biology of the Cell. 9 (9): 2393–3405. PMC 25506
 . PMID 9725902. doi:10.1091/mbc.9.9.2393.
. PMID 9725902. doi:10.1091/mbc.9.9.2393. - ↑ X. Escote; M. Zapater; J. Clotet; F. Posas (2004). "Hog1 mediates cell-cycle arrest in G1 phase by the dual targeting of Sic1". Nat Cell Biol. 6 (10): 997–1002. PMID 15448699. doi:10.1038/ncb1174.
- ↑ P. Coccetti; V. Zinzalla; G. Tedeschi; G.L. Russo; S. Fantinato; O. Marin; L.A. Pinna; M. Vanoni; L. Alberghina (2006). "Sic1 is phosphorylated by CK2 on Ser201 in budding yeast cells". Biochemical and Biophysical Research Communications. 346 (3): 786–793. PMID 16777072. doi:10.1016/j.bbrc.2006.05.171.
- ↑ Barberis M, De Gioia L, Ruzzene M, et al. (May 2005). "The yeast cyclin-dependent kinase inhibitor Sic1 and mammalian p27Kip1 are functional homologues with a structurally conserved inhibitory domain". Biochem. J. 387 (Pt 3): 639–47. PMC 1134993
 . PMID 15649124. doi:10.1042/BJ20041299.
. PMID 15649124. doi:10.1042/BJ20041299. - ↑ M.L.C. Khoo; J.L. Freeman; I.J. Witterick; J.C. Irish; L.E. Rotstein; P.J. Gullane; S.L. Asa (2002). "Underexpression of p27/Kip in thyroid papillary microcarcinomas with gross metastatic disease.". Arch Otolaryngol Head Neck Surg. 128 (3): 253–257. PMID 11886339. doi:10.1001/archotol.128.3.253.
- ↑ D.J. Mann; E.S. Child; C. Swanton; H. Laman; N. Jones (1999). "Modulation of p27Kip1 levels by the cyclin encoded by Kaposi's sarcoma-associated herpesvirus". The EMBO Journal. 18 (3): 654–663. PMC 1171158
 . PMID 9927425. doi:10.1093/emboj/18.3.654.
. PMID 9927425. doi:10.1093/emboj/18.3.654. - ↑ D. Nitti; C. Belluco; E. Mammano; A. Marchet; A. Ambrosi; R. Mencarelli; P. Segato; M. Lise (2002). "Low level of p27(Kip1) protein expression in gastric adenocarcinoma is associated with disease progression and poor outcome". Journal of Surgical Oncology. 81 (4): 167–176. PMID 12451619. doi:10.1002/jso.10172.