Shikimic acid
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
(3R,4S,5R)-3,4,5-trihydroxycyclohex-1-ene-1-carboxylic acid | |||
| Identifiers | |||
| 3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.850 | ||
| EC Number | 205-334-2 | ||
| KEGG | |||
| PubChem CID |
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C7H10O5 | |||
| Molar mass | 174.15 g/mol | ||
| Melting point | 185 to 187 °C (365 to 369 °F; 458 to 460 K) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Shikimic acid, more commonly known as its anionic form shikimate, is a cyclohexene, a cyclitol and a cyclohexanecarboxylic acid. It is an important biochemical metabolite in plants and microorganisms. Its name comes from the Japanese flower shikimi (シキミ, the Japanese star anise, Illicium anisatum), from which it was first isolated in 1885 by Johan Fredrik Eykman.[1] The elucidation of its structure was made nearly 50 years later.[2]
Properties
It appears in the list of Group 3 carcinogens of the International Agency for Research on Cancer. Group 3 means that the agent is not classifiable as to its carcinogenicity to humans. Nevertheless, it is recommended to roast tree fern fronds, a specialty called fiddlehead (furled fronds of a young tree fern in the order Cyatheales, harvested for use as a vegetable). These fronds are edible, but must be roasted first to remove shikimic acid.[3]
Shikimic acid is also the glycoside part of some hydrolysable tannins. The acid is highly soluble in water and insoluble in nonpolar solvents, and this reflects on why shikimic acid is active only against gram-positive bacteria, due to outer membrane impermeability of gram-negatives.[4]
Biosynthesis
Phosphoenolpyruvate and erythrose-4-phosphate react to form 3-deoxy-D-arabinoheptulosonate-7-phosphate (DAHP), in a reaction catalyzed by the enzyme DAHP synthase. DAHP is then transformed to 3-dehydroquinate (DHQ), in a reaction catalyzed by DHQ synthase. Although this reaction requires nicotinamide adenine dinucleotide (NAD) as a cofactor, the enzymic mechanism regenerates it, resulting in the net use of no NAD.
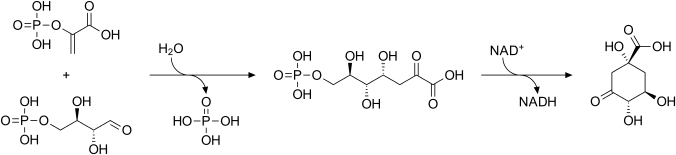 Biosynthesis of 3-dehydroquinate from Phosphoenolpyruvate and erythrose-4-phosphate
Biosynthesis of 3-dehydroquinate from Phosphoenolpyruvate and erythrose-4-phosphate
DHQ is dehydrated to 3-dehydroshikimic acid by the enzyme 3-dehydroquinate dehydratase, which is reduced to shikimic acid by the enzyme shikimate dehydrogenase, which uses nicotinamide adenine dinucleotide phosphate (NADPH) as a cofactor.
 Biosynthesis of shikimic acid from 3-dehydroquinate
Biosynthesis of shikimic acid from 3-dehydroquinate
Shikimate pathway
Biosynthesis of the aromatic amino acids
The shikimate pathway is a seven step metabolic route used by bacteria, fungi, algae, parasites, and plants for the biosynthesis of aromatic amino acids (phenylalanine, tyrosine, and tryptophan). This pathway is not found in animals; therefore, phenylalanine and tryptophan represent essential amino acids that must be obtained from the animal's diet (animals can synthesise tyrosine from phenylalanine, and therefore is not an essential amino acid except for individuals unable to hydroxylate phenylalanine to tyrosine).
The seven enzymes involved in the shikimate pathway are DAHP synthase, 3-dehydroquinate synthase, 3-dehydroquinate dehydratase, shikimate dehydrogenase, shikimate kinase, EPSP synthase, and chorismate synthase. The pathway starts with two substrates, phosphoenol pyruvate and erythrose-4-phosphate and ends with chorismate, a substrate for the three aromatic amino acids. The fifth enzyme involved is the shikimate kinase, an enzyme that catalyzes the ATP-dependent phosphorylation of shikimate to form shikimate 3-phosphate (shown in the figure below).[5] Shikimate 3-phosphate is then coupled with phosphoenol pyruvate to give 5-enolpyruvylshikimate-3-phosphate via the enzyme 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase.
Then 5-enolpyruvylshikimate-3-phosphate is transformed into chorismate by a chorismate synthase.
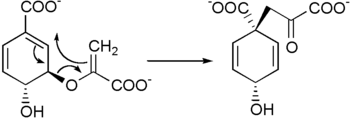
Prephenic acid is then synthesized by a Claisen rearrangement of chorismate by Chorismate mutase.[6][7]
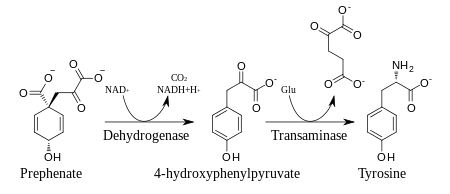
Prephenate is oxidatively decarboxylated with retention of the hydroxyl group to give p-hydroxyphenylpyruvate, which is transaminated using glutamate as the nitrogen source to give tyrosine and α-ketoglutarate.
Starting point in the biosynthesis of some phenolics
Phenylalanine and tyrosine are the precursors used in the phenylpropanoids biosynthesis. The phenylpropanoids are then used to produce the flavonoids, coumarins, tannins and lignin. The first enzyme involved is phenylalanine ammonia-lyase (PAL) that converts L-phenylalanine to trans-cinnamic acid and ammonia.
- Gallic acid biosynthesis
Gallic acid is formed from 3-dehydroshikimate by the action of the enzyme shikimate dehydrogenase to produce 3,5-didehydroshikimate. This latter compound spontaneously rearranges to gallic acid.[8]
Other compounds
Shikimic acid is a precursor for:
- indole, indole derivatives and aromatic amino acid tryptophan and tryptophan derivatives such as the psychedelic compound dimethyltryptamine
- many alkaloids and other aromatic metabolites
Mycosporine-like amino acids
Mycosporine-like amino acids are small secondary metabolites produced by organisms that live in environments with high volumes of sunlight, usually marine environments.
Uses
In the pharmaceutical industry, shikimic acid from the Chinese star anise (Illicium verum) is used as a base material for production of oseltamivir (Tamiflu). Although shikimic acid is present in most autotrophic organisms, it is a biosynthetic intermediate and in general found in very low concentrations. The low isolation yield of shikimic acid from the Chinese star anise is blamed for the 2005 shortage of oseltamivir. Shikimic acid can also be extracted from the seeds of the sweetgum (Liquidambar styraciflua) fruit,[2] which is abundant in North America, in yields of around 1.5%. For example, 4 kg of sweetgum seeds is needed for fourteen packages of Tamiflu. By comparison, star anise has been reported to yield 3 to 7% shikimic acid. Biosynthetic pathways in E. coli have recently been enhanced to allow the organism to accumulate enough material to be used commercially.[9][10][11] A 2010 study released by the University of Maine showed that shikimic acid can also be readily harvested from the needles of several species of pine tree.[12]
Protecting groups are more commonly used in small-scale laboratory work and initial development than in industrial production processes because their use adds additional steps and material costs to the process. However, the availability of a cheap chiral building block can overcome these additional costs e.g. shikimic acid for oseltamivir.
Aminoshikimic acid is also an alternative to shikimic acid as a starting material for the synthesis of oseltamivir.
Target for drugs
Shikimate can be used to synthesise (6S)-6-Fluoroshikimic acid,[13] an antibiotic which inhibits the aromatic biosynthetic pathway.[14]
Glyphosate, the active ingredient in the herbicide Roundup, kills plants by interfering with the shikimate pathway in plants. More specifically, glyphosate inhibits the enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS). "Roundup Ready" genetically modified crops overcome that inhibition.
See also
- Aminoshikimate pathway, a novel variation of the shikimate pathway
Books
- The shikimate pathway, E. Haslam, 2 editions - first published in 1974
- Shikimic acid, E. Haslam, 1 edition - first published in 1993
References
- ↑ The Botanical Relations of Illicium Religiosum, Sieb., Illicium Anisatum, Lour. J. F. Eykman, American Journal of Pharmacy, 1881, volume 53, Number 8 (article)
- 1 2 Liquidambar styraciflua: a renewable source of shikimic acid. Liza B. Enrich, Margaret L. Scheuermann, Ashley Mohadjer, Kathryn R. Matthias, Chrystal F. Eller, M. Scott Newman, Michael Fujinaka and Thomas Poon, Tetrahedron Letters, 2008, volume 49, pages 2503–2505, doi:10.1016/j.tetlet.2008.02.140
- ↑ Carcinogenicity of bracken and shikimic acid. I. A. Evans and M. A. Osman, Nature, 26 July 1974, volume 250, pages 348 - 349, doi:10.1038/250348a0
- ↑ http://ocean.kisti.re.kr/downfile/volume/kfn/E1FSA3/2009/v14n3/E1FSA3_2009_v14n3_195.pdf
- ↑ Herrmann, K. M.; Weaver, L. M. (1999). "The Shikimate Pathway". Annual Review of Plant Physiology and Plant Molecular Biology. 50: 473–503. PMID 15012217. doi:10.1146/annurev.arplant.50.1.473.
- ↑ Helmut Goerisch (1978). "On the mechanism of the chorismate mutase reaction". Biochemistry. 17 (18): 3700. doi:10.1021/bi00611a004.
- ↑ Peter Kast, Yadu B. Tewari, Olaf Wiest, Donald Hilvert, Kendall N. Houk, and Robert N. Goldberg (1997). "Thermodynamics of the Conversion of Chorismate to Prephenate: Experimental Results and Theoretical Predictions". J. Phys. Chem. B. 101 (50): 10976–10982. doi:10.1021/jp972501l.
- ↑ Gallic acid pathway on metacyc.org
- ↑ Bradley, David (December 2005). "Star role for bacteria in controlling flu pandemic?". Nature Reviews Drug Discovery. 4 (12): 945–946. PMID 16370070. doi:10.1038/nrd1917. Retrieved 2007-03-07.
- ↑ Marco Krämer; Johannes Bongaertsa; Roel Bovenberga; Susanne Kremera; Ulrike Müllera; Sonja Orfa; Marcel Wubboltsa; Leon Raevena. (2003). "Metabolic engineering for microbial production of shikimic acid". Metabolic Engineering. 5 (4): 277–283. PMID 14642355. doi:10.1016/j.ymben.2003.09.001.
- ↑ Johansson Louise; Lindskog Anna; Silfversparre Gustav; Cimander Christian; Nielsen Kristian Fog; Liden Gunnar (2005). "Shikimic acid production by a modified strain of E. coli (W3110.shik1) under phosphate-limited and carbon-limited conditions". Biotechnology and Bioengineering. 92 (5): 541–552. PMID 16240440. doi:10.1002/bit.20546.
- ↑ Maine pine needles yield valuable Tamiflu material, Boston.com, November 7, 2010
- ↑ http://jiang.tju.edu.cn/pdfs/6flufull.pdf
- ↑ http://aac.asm.org/content/38/2/403.full.pdf



