Sesquiterpene

Sesquiterpenes are a class of terpenes that consist of three isoprene units and have the Molecular formula C15H24. Like monoterpenes, sesquiterpenes may be acyclic or contain rings, including many unique combinations. Biochemical modifications such as oxidation or rearrangement produce the related sesquiterpenoids.
Sesquiterpenes are found naturally in plants and insects, as semiochemicals, e.g. defensive agents or pheromones.
Acyclic
The reaction of geranyl pyrophosphate with isopentenyl pyrophosphate results in the 15-carbon farnesyl pyrophosphate which is an intermediate in the biosynthesis of sesquiterpenes such as farnesene. Oxidation of farnesene then provides sesquiterpenoids such as farnesol.
Monocyclic
There are more cyclic sesquiterpenes than cyclic monoterpenes because of the increased chain length and additional double bond. In addition to common six-membered ring systems such as is found in zingiberene (a constituent of the oil from ginger), cyclization of one end of the chain to the other end can lead to macrocyclic rings such as humulene.
Bicyclic
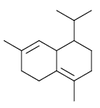
The cadinenes contain two fused six-membered rings. Caryophyllene, a component of many essential oils such as clove oil, contains a nine-membered ring fused to a cyclobutane ring.
Vetivazulene and guaiazulene are aromatic bicyclic sesquiterpenoids.
Tricyclic
With the addition of a third ring, the possible structures become increasingly varied. Examples include longifolene, copaene and the alcohol patchoulol.
Dictyophorine A and B
Two sesquiterpenes named dictyophorine A and B were identified from the fungus Phallus indusiatus. These compounds are based on the eudesmane skeleton (a common structure found in plant-derived flavors and fragrances), and they are the first eudesmane derivatives isolated from fungi. The dictyophorines promote the synthesis of nerve growth factor in astroglial cells.[1]
Footnotes
- ↑ Kawagishi, Hirokazu (July 1997). "Dictyophorines A and B, two stimulators of NGF-synthesis from the mushroom Dictyophora indusiata". Phytochemistry. Elsevier. 45 (6): 1203–1205. PMID 9272967. doi:10.1016/s0031-9422(97)00144-1.
External links
- Sesquiterpenes at the US National Library of Medicine Medical Subject Headings (MeSH)