Separatory funnel
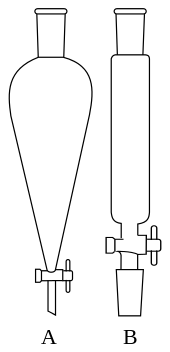
A separatory funnel, also known as separation funnel, separating funnel, or colloquially sep funnel, is a piece of laboratory glassware used in liquid-liquid extractions to separate (partition) the components of a mixture into two immiscible solvent phases of different densities.[1] Typically, one of the phases will be aqueous, and the other a lipophilic organic solvent such as ether, MTBE, dichloromethane, chloroform, or ethyl acetate. All of these solvents form a clear delineation between the two liquids.[2] The more dense liquid, typically the aqueous phase unless the organic phase is halogenated, sinks and can be drained out through a valve away from the less dense liquid, which remains in the separatory funnel.[3]
Description

A separating funnel takes the shape of a cone with a hemispherical end. It has a stopper at the top and stopcock (tap), at the bottom. Separating funnels used in laboratories are typically made from borosilicate glass and their stopcocks are made from glass or PTFE. Typical sizes are between 30 mL and 3 L. In industrial chemistry they can be much bigger and for much larger volumes centrifuges are used. The sloping sides are designed to facilitate the identification of the layers. The stopcock-controlled outlet is designed to drain the liquid out of the funnel. On top of the funnel there is a standard taper joint which fits with a ground glass or Teflon stopper.[4]
To use a separatory funnel, the two phases and the mixture to be separated in solution are added through the top with the stopcock at the bottom closed. The funnel is then closed and shaken gently by inverting the funnel multiple times; if the two solutions are mixed together too vigorously emulsions will form. The funnel is then inverted and the tap carefully opened to release excess vapor pressure. The separating funnel is set aside to allow for the complete separation of the phases. The top and the bottom tap are then opened and the lower phase is released by gravitation. The top must be opened while releasing the lower phase to allow pressure equalization between the inside of the funnel and the atmosphere. When the bottom layer has been removed, the stopcock is closed and the upper layer is poured out through the top into another container.
Theory
The separatory funnel runs on the concept of "like dissolves like", with different solutes being preferentially soluble in certain solvents. While a separatory funnel is being shaken, the two solvents mix and share a large surface area, which allows each solute to migrate to the solvent in which it is more soluble.
The solvents do not form a unified solution together because they are immiscible. When the funnel is allowed to sit after being shaken, the liquids form distinct physical layers, with the less dense liquid floating and more dense sinking. A mixture of solutes is thus separated into two physically separate solutions, each enriched in different solutes.
The bottom layer is drained out, then the top layer can be retained in the separatory funnel for further extractions with additional batches of solvent or drained out into a separate vessel for other uses. If it is desired to retain the bottom layer in the separatory funnel for further extractions, both layers are taken out separately, and then the former bottom layer is returned to the separatory funnel.
Each independent solution can then be extracted again with additional batches of solvent, used for other physical or chemical processes. If the goal was to separate a soluble material from mixture, the solution containing that desired product can sometimes simply be evaporated to leave behind the purified solute. For this reason, it is a practical benefit to use volatile solvents for extracting the desired material from the mixture.[5]
Emulsions
One of the drawbacks of using a separatory funnel is emulsions can form easily, and can take a long time to separate once formed. They are often formed while liquids are being mixed in the separatory funnel. This can occur when small droplets are suspended in an aqueous solution. If an emulsion is formed, one technique used to separate the liquids is to slowly swirl the solution in the separatory funnel. If the emulsion is not separated by this process, a small amount of saturated saline solution is added.[6]
Research is being done on alternative, more efficient techniques, mostly utilizing stir bars to decrease or even eliminate the chance of emulsification, thus decreasing the amount of waiting time.[7]
Safety concerns
The largest risk when using a separatory funnel is that of pressure build-up. Pressure accumulates during mixing if a gas evolving reaction or physical change occurs. This problem can be easily handled by simply opening the stopper at the top of the funnel routinely while mixing. This should be done with the top of the funnel pointed away from the body.[8]
Gallery

- Upper phase
- Lower phase
 The ether layer with a dissolved yellow compound is on top and an aqueous layer is at the bottom
The ether layer with a dissolved yellow compound is on top and an aqueous layer is at the bottom
See also
- Dropping funnels are similar in shape and design, and may be used as separatory funnels. They have standard taper ground glass joints at the stem.
- Partition coefficient is a measure of the distribution of an analyte between the two phases in a separation.
- Decantation is a process of pouring the top layer off of a bottom layer of liquid or solid
References
- ↑ http://www.tutorvista.com/content/chemistry/chemistry-i/elements-compounds/separating-funnel-use.php
- ↑ http://chem.allegheny.edu/chem231/sep%20funnel%20primer.pdf
- ↑ Padìas, Anne B. (2011). Making the Connections2: A How-To Guide for Organic Chemistry Lab Techniques.Plymouth, Michigan: Hayden-McNeil Publishing, p. 129.
- ↑ http://orgchem.colorado.edu/equipment/glassware/sepfun.html
- ↑ https://www.erowid.org/archive/rhodium/chemistry/equipment/recrystallization.html
- ↑ http://www.uwplatt.edu/chemep/chem/chemscape/labdocs/catofp/mixpour/sepfunl/sepfunl.htm
- ↑ "A novel and fast extraction technique as an alternative to conventional separatory funnels". Fresenius' Journal of Analytical Chemistry. 345: 411–414. doi:10.1007/BF00325616.
- ↑ Fessenden, J., Joan S. Fessenden, Patty Feist. Organic Laboratory Techniques, 3rd Edition, 2001. Pacific Grove, California: Brooks Cole Publishing, p. 59.