Scar free healing
Scar free healing is the process by which significant injuries can heal without permanent damage to the tissue the injury has affected. In most healing, scars form due to the fibrosis and wound contraction, however in scar free healing tissue is completely regenerated. Scar improvement, and scar-free healing are an important and relevant area of medicine. During the 90s, published research on the subject increased; it's a relatively recent term in the literature. Scar free healing is something which takes place in foetal life but the capacity is lost during progression to adulthood. In amphibians, tissue regeneration occurs, for example, as in skin regeneration in the adult axolotl.[1]
Scar versus scar free healing
Scarring takes place in response to damaged or missing tissue following injury due to biological processes or wounding, it is a process that occurs in order to replace the lost tissue.[2] The process of scarring is complex, it involves the inflammatory response and remodelling amongst other cell activities. Many growth factors and cytokines are also involved in the process, as well as extracellular matrix interactions.[2] Mast cells are one cell type which act to promote scarring.[3]
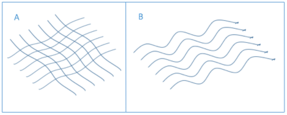
Scarring during healing can create both physical and psychological problems, and is a significant clinical burden which is why the scar free healing concept is of interest. Some of the problems in scar healing lie in the physical outcome of the process: for example when collagen is abnormally organised in scar tissue. In scars the collagen is arranged in parallel bundles of the collagen fibres whilst healthy scar free tissue has a "basket weave" structure (Figure 1).[2] The difference in collagen arrangement along with a lack of difference in the dermal tissue when healing has taken place with or without scarring is indicative of regenerative failure of normal skin.[2] Severe scarring resulting from these collagen deposits is known as hypertrophic scarring and is of great concern worldwide with an incidence ranging from 32–72%.[4]
Scar free healing in nature
Unlike the limited regeneration seen in adult humans, many animal groups possess an ability to completely regenerate damaged tissue.[5] Full limb regeneration is seen both in invertebrates (e.g. starfish and flatworms which can regenerate fully functioning appendages) and some vertebrates, however in the latter this is almost always confined to the immature members of the species: an example being tadpoles which can regrow their tails and various other body parts, an ability not seen in the mature frogs.[6] The exception to this is the much studied urodele species' of amphibians, also known as salamanders, which carry their ability of complete regeneration into adulthood.[2] These vertebrates possess an exceptional ability to allow regeneration of entire limbs and their tails (as well as a multitude of their internal organs as well, including their spinal cord)[2] through a process known as blastema formation.[7] This involves covering of the wound by a layer of epithelial cells known as the wound cap and subsequent innervation of this area with nerves that give off signals that revert local differentiated cells (such as muscle, cartilage and connective tissue) back to their undifferentiated cell lineage also known as mesenchymal cells.[7] It is this area that is known as the blastema which has the potential to differentiate and proliferate once again allowing regrowth of the limb similar to how it occurs during development.[8] In wound-healing in urodeles it is the quick response of anti-inflammatory macrophages which have been shown to be key to their regeneration capabilities. In one study, it was found that limbs would not regenerate in those urodeles with depleted macrophages and instead would scar with permanent loss of functionality.[9] Knowing how regeneration occurs in animals such as these may have great implications for how wound-healing is tackled in medicine and research has been aimed at this area, as a result.
Fetal vs adult healing in humans
Reparation of tissue in the mammalian fetus is radically different than the healing mechanisms observed in a healthy adult. During early gestation fetal skin wounds have the remarkable ability to heal rapidly and without scar formation. Wound healing itself is a particularly complex process and the mechanisms by which scarring occurs involves inflammation, fibroplasia, the formation of granulation tissue and finally scar maturation. Since the observation of scar free healing was first reported in the early fetus more than three decades ago, research has focused intently on the underlying mechanisms which separate scarless fetal wound repair from normal adult wound healing.
Scar free healing has been documented in fetuses across the animal kingdom, including mice, rats, monkeys, pigs and humans. It is important to note that the ability of fetuses to heal without scarring is wound size dependent and also age-dependent, whereby after a specific gestational age, usually 24 weeks in humans, typical scar formation will occur. While the exact mechanisms of scar free healing in the fetus remain unknown, research has shown that it is thought to be due to the complex interaction of the components of the extracellular matrix (ECM), the inflammatory response, cellular mediators and the expression of specific growth factors.[10]
Intrauterine environment
Originally, it was thought that the intrauterine environment, the sterile amniotic fluid surrounding the embryo, was responsible for fetal scar free healing. Reasoning that embryonic wounds healed scarlessly because they were not exposed to the same contaminating agents which normal adult wounds were exposed to such as bacteria and viruses. However this theory was discredited by investigating fetal wound healing in the pouch of a young marsupial. These pouches can often be exposed to maternal faeces and urine, a highly different environment to the sterile intrauterine environment seen in eutherian embryos. Despite these differences skin wounds on the marsupial healed without the formation of a scar, proving the irrelevance of the embryonic environment in scar free healing.
Immune system cells and inflammatory response
One of the major differences between embryonic scar-free healing wounds and adult scar-forming wounds is the role played by the cells of the immune system and the inflammatory response.
Table 1: Summary of the major differences identified between fetal and adult wound healing.[11][12]
| Select Component | Fetal | Adult | Role in Wound Healing | |
| Immune System and Inflammation | IL-10
IL-6/8 |
High levels
Low levels |
Low levels
High levels |
Anti-inflammatory cytokines
Pro-inflammatory cytokines |
| Extracellular Matrix (ECM) | Hyaluronic acid
CD44 (hyaluronic acid receptor Tenascin Fibronectin Decorin Fibromodulin Collagen |
High levels
High levels High levels High levels Low levels High levels Elevated ratio of type 111 to type 1 |
Low levels
Low levels Low levels Low levels High levels Low levels Elevated ratio of type 1 to type 111 |
Cellular movement, cell-matrix interactions, cell migration
Anti-adhesive, anti-proliferative Tissue architecture, cell proliferation/migration, cell matrix interactions Inhibits fibrillogenesis Tissue architecture, ECM remodeling, tensile strength, cell-matrix interactions |
| Growth Factors | EGF
PDGF FGF TGF-β1 TGF-β2 TGF-β3 VEGF |
High levels
Low levels Low levels Low levels Low levels High levels Low levels |
Decreases with age
High levels High levels High levels High levels Low levels High levels |
Stimulate fibroblasts to secrete collagen
Fibroplasia Matrix deposition, fibroblast migration, angiogenesis Infiltration of neutrophils and macrophages, fibroplasia, scarring, fibrosis Infiltration of neutrophils and macrophages, fibroplasia, scarring, fibrosis Possible role in anti-scarring Angiogenesis |
| Wound Closure | Actin cable | Myofibroblasts |
The fetal immune system can be described as 'immunologically immature' due to the marked reduction in neutrophils, macrophages, monocytes, lymphocytes and also inflammatory mediators, compared with adult wounds.[13] Physiologically, adult and fetal neutrophils differ, due to the fact that the concentration of neutrophils is higher in the adult than the fetus, this results in phagocytosis of the wound and the recruitment and release of inflammatory cytokines. Leading to the promotion of a more aggressive inflammatory response in adult wound healing. It is also thought that the time in which this inflammatory response occurs, is much shorter in the fetus thus limiting any damage.[14]
Role of the extracellular matrix and its components
Another difference between the healing of embryonic and adult wounds is due to the role of fibroblast cells. Fibroblasts are responsible for the synthesis of the ECM and collagen. In the fetus, fibroblasts are able to migrate at a faster rate than those found in the adult wound. Fetal fibroblasts can also proliferate and synthesize collagen simultaneously, in comparison to adult fibroblasts where collagen synthesis is delayed. It is this delay in both collagen deposition and migration, which is likely to contribute to formation of a scar in the adult.
Proteins and cell surface receptors found in the ECM differ in fetal and adult wound healing. This is due to the early up regulation of cell adhesion proteins such as fibronectin and tenascin in the fetus. During early gestation in the fetal wounds of rabbits, the production of fibronectin occurs around 4 hours after wounding, much faster than in adult wounds where expression of fibronectin does not occur until 12 hours post wounding. The same pattern can be seen in the deposition of tenascin. It is this ability of the fetal fibroblast to quickly express and deposit fibronectin and tenascin, which ultimately allows cell migration and attachment to occur, resulting in an organised matrix with less scarring.[14]
Another major component of the ECM is hyaluronic acid (HA), a glycosaminoglycan. It is known that fetal skin contains more HA than adult skin due to the expression of more HA receptors. The expression of HA is known to down-regulate the recruitment of inflammatory cytokines interleukin-1 (IL-1) and tumour necrosis factor-alpha (TNF-α); since fetal wounds contain a reduced number of pro-inflammatory mediators than adult wounds it is thought that the higher levels of HA in the fetal skin aid in scar free healing.
Analysis using microarrays has also shown that gene expression profiles greatly differ between scar free fetal wounds and postnatal wounds with scar formation. In scarlesss wound healing there is a significant up-regulation in genes associated with cell growth and proliferation, thought to be a major contributing factor to the rapid wound closure seen in the foetus.[10] Whilst wound healing in the fetus has been shown to be completely scarless in an age-dependent manner, adult mammals do not have complete scar free healing but have retained some regenerative properties. Adult regeneration is limited to a number of organs, most notably, the liver.
Continued regeneration in adult humans
There are few examples of regeneration in humans continuing after fetal life in to adulthood. Generally, adult wound healing involves fibrotic processes causing wound contraction which may lead to the formation of scar tissue.[15] In regeneration, however, completely new tissue is synthesized. This can lead to scar free healing where the function and structure of the organ is reinstated.[16] However organ regeneration is not yet fully understood.
Two types of regeneration in human adults are currently recognised; spontaneous and induced.[2]
Spontaneous regeneration occurs in the human body naturally. The most recognised example of this is the regeneration of the liver,[17] which can regenerate up to two thirds of its mass when injured by surgical removal, ischaemia or after exposure to harmful toxins.[17] (Figure 2)

Through this mechanism the liver can be restored to its original state, scar-free. However, despite nearly 80 years of research on liver regeneration much debate still surrounds the exact mechanisms by which the process occurs.[17]
Another example of spontaneous regeneration endometrial lining of the uterus after menses during reproductive years. Endometrial glands from a basal layer of the uterine wall can regenerate the functional layer without fibrosis or scarring.[18]
Most recently, the kidney has been found to have the ability to regenerate. Following removal or incapacitation of one kidney the other may double in size in order to counteract the loss of the other kidney. This is known a compensatory growth.[19]
Induced regeneration stimulated by an outside source of a "non-regenerative" organ.[2] In humans is for therapeutic use. Induced regeneration iscurrently being trialled to replace organ transplants as issues such as rejection, lack of donors and scarring would be eliminated.[20]
The table below details some of the tissues in which induced regeneration has been attempted;
| Tissue | Type of Regeneration | Mechanisms of Regeneration and current research tools |
| Heart Muscle | Induced | Using differentiation of somatic stem cells into cardiomyocytes.[21] |
| Thymus | Induced | Up regulating FOXN1, which causes increased expression of thymic epithelial cell specific receptor, which regenerates an aged thymus.[22] |
| Vagina | Induced | Reconstruction of vaginal muscle and epithelial cells using biodegradable scaffolds.[23] |
| Skin | Induced | Use of a regeneratively active collagen scaffold to prevent wound contraction.[2] |
| Peripheral Nerve | Induced | Use of a regeneratively active collagen scaffold to prevent wound contraction.[2] |
Clinical burden and implications of scarring
Following injury or surgery, a doctor's key aim is to restore full function in a patient and help ensure they return to as close to their original state before their skin trauma or surgery.[24] Ensuring patients return as closely to their original appearance and original function is challenging in the context of scarring. Scar-free healing is yet to be observed in healthy post gestational humans, despite being seen in human embryos. Currently, it is only possible to reduce scar visibility, and the NHS suggests a number of different methods of doing this including corticosteroid injections, skin creams, silicone gels, pressure dressings, dermal fillers, radiotherapy and laser therapy.[25] Although these methods do reduce a scars visible appearance, they do not result in a scar free appearance. Billions of pounds is spent on wound maintenance and healing on the NHS every year. Between 2014 and 2015 in England and Wales, 19,239 people sustained a burn injury which required hospital care.[26] In addition to the significant financial cost, the cost of scars is immense to the patients too. One study into the quality of life of patients with scars found that over half of the participants felt stigmatised by their scars and felt their personal relationships deteriorated. In addition to this, 68% tried to hide their scars, whilst reporting their work life, self-confidence and ability to communicate with others had been negatively affected.[27] Future research and advances in scar-free healing could lessen the cost to the NHS whilst also improving the quality of life to many people affected.
References
- ↑ Seifert, Ashley W.; Monaghan, James R.; Voss, S. Randal; Maden, Malcolm (2012-01-01). "Skin regeneration in adult axolotls: a blueprint for scar-free healing in vertebrates". PloS One. 7 (4): e32875. ISSN 1932-6203. PMC 3317654
 . PMID 22485136. doi:10.1371/journal.pone.0032875.
. PMID 22485136. doi:10.1371/journal.pone.0032875. - 1 2 3 4 5 6 7 8 9 10 Occleston, Nick L.; Metcalfe, Anthony D.; Boanas, Adam; Burgoyne, Nicholas J.; Nield, Kerry; O'Kane, Sharon; Ferguson, Mark W. J. (2010-01-01). "Therapeutic improvement of scarring: mechanisms of scarless and scar-forming healing and approaches to the discovery of new treatments". Dermatology Research and Practice. 2010: 1–10. ISSN 1687-6113. PMC 2929503
 . PMID 20811598. doi:10.1155/2010/405262.
. PMID 20811598. doi:10.1155/2010/405262. - ↑ Ud-Din, Sara; Volk, Susan W.; Bayat, Ardeshir (2014-09-01). "Regenerative healing, scar-free healing and scar formation across the species: current concepts and future perspectives". Experimental Dermatology. 23 (9): 615–619. ISSN 1600-0625. PMID 24863070. doi:10.1111/exd.12457.
- ↑ Gangemi, Ezio Nicola; Gregori, Dario; Berchialla, Paola; Zingarelli, Enrico; Cairo, Monica; Bollero, Daniele; Ganem, Jamal; Capocelli, Roberto; Cuccuru, Franca (2008-04-01). "Epidemiology and risk factors for pathologic scarring after burn wounds". Archives of Facial Plastic Surgery. 10 (2): 93–102. ISSN 1521-2491. PMID 18347236. doi:10.1001/archfaci.10.2.93.
- ↑ Bryant, Susan V.; Endo, Tetsuya; Gardiner, David M. (2002-01-01). "Vertebrate limb regeneration and the origin of limb stem cells". The International Journal of Developmental Biology. 46 (7): 887–896. ISSN 0214-6282. PMID 12455626.
- ↑ Godwin, James W.; Rosenthal, Nadia (2014-01-01). "Scar-free wound healing and regeneration in amphibians: Immunological influences on regenerative success". Differentiation. Exotic Animals in Development. 87 (1–2): 66–75. doi:10.1016/j.diff.2014.02.002.
- 1 2 Brockes, Jeremy P.; Kumar, Anoop (2002-08-01). "Plasticity and reprogramming of differentiated cells in amphibian regeneration". Nature Reviews Molecular Cell Biology. 3 (8): 566–574. ISSN 1471-0072. PMID 12154368. doi:10.1038/nrm881.
- ↑ Roy, Stéphane; Lévesque, Mathieu (2006-01-01). "Limb regeneration in axolotl: is it superhealing?". TheScientificWorldJournal. 6 Suppl 1: 12–25. ISSN 1537-744X. PMID 17205184. doi:10.1100/tsw.2006.324.
- ↑ Godwin, James W.; Pinto, Alexander R.; Rosenthal, Nadia A. (2013-06-04). "Macrophages are required for adult salamander limb regeneration". Proceedings of the National Academy of Sciences. 110 (23): 9415–9420. ISSN 0027-8424. PMC 3677454
 . PMID 23690624. doi:10.1073/pnas.1300290110.
. PMID 23690624. doi:10.1073/pnas.1300290110. - 1 2 Larson, Barrett J.; Longaker, Michael T.; Lorenz, H. Peter. "Scarless Fetal Wound Healing: A Basic Science Review". Plastic and Reconstructive Surgery. 126 (4): 1172–1180. PMC 4229131
 . PMID 20885241. doi:10.1097/prs.0b013e3181eae781.
. PMID 20885241. doi:10.1097/prs.0b013e3181eae781. - ↑ Yates, Cecelia C.; Hebda, Patricia; Wells, Alan (2012-12-01). "Skin Wound Healing and Scarring: Fetal Wounds and Regenerative Restitution". Birth Defects Research Part C: Embryo Today: Reviews. 96 (4): 325–333. ISSN 1542-9768. PMC 3967791
 . PMID 24203921. doi:10.1002/bdrc.21024.
. PMID 24203921. doi:10.1002/bdrc.21024. - ↑ Rolfe, K. J.; Grobbelaar, A. O. (2012-05-17). "A Review of Fetal Scarless Healing". ISRN Dermatology. 2012: 1–9. PMC 3362931
 . PMID 22675640. doi:10.5402/2012/698034.
. PMID 22675640. doi:10.5402/2012/698034. - ↑ Ferguson, Mark W. J.; O'Kane, Sharon (2004-05-29). "Scar–free healing: from embryonic mechanisms to adult therapeutic intervention". Philosophical Transactions of the Royal Society of London B: Biological Sciences. 359 (1445): 839–850. ISSN 0962-8436. PMC 1693363
 . PMID 15293811. doi:10.1098/rstb.2004.1475.
. PMID 15293811. doi:10.1098/rstb.2004.1475. - 1 2 Lo, David D.; Zimmermann, Andrew S.; Nauta, Allison; Longaker, Michael T.; Lorenz, H. Peter (2012-09-01). "Scarless fetal skin wound healing update". Birth Defects Research Part C: Embryo Today: Reviews. 96 (3): 237–247. ISSN 1542-9768. doi:10.1002/bdrc.21018.
- ↑ Gurtner, Geoffrey C.; Werner, Sabine; Barrandon, Yann; Longaker, Michael T. (2008-05-15). "Wound repair and regeneration". Nature. 453 (7193): 314–321. ISSN 0028-0836. PMID 18480812. doi:10.1038/nature07039.
- ↑ Tissue and Organ Regeneration in Adults - Extension of the | Ioannis V. Yannas | Springer.
- 1 2 3 "Liver Regeneration - ScienceDirect". www.sciencedirect.com. Retrieved 2016-09-28.
- ↑ Gargett, Caroline E.; Nguyen, Hong P. T.; Ye, Louie (2012-12-01). "Endometrial regeneration and endometrial stem/progenitor cells". Reviews in Endocrine & Metabolic Disorders. 13 (4): 235–251. ISSN 1573-2606. PMID 22847235. doi:10.1007/s11154-012-9221-9.
- ↑ Fong, Debra; Denton, Kate M.; Moritz, Karen M.; Evans, Roger; Singh, Reetu R. (2014-03-01). "Compensatory responses to nephron deficiency: adaptive or maladaptive?". Nephrology (Carlton, Vic.). 19 (3): 119–128. ISSN 1440-1797. PMID 24533732. doi:10.1111/nep.12198.
- ↑ Yannas, Ioannis V. (2005-12-22). "Similarities and differences between induced organ regeneration in adults and early foetal regeneration". Journal of the Royal Society, Interface / the Royal Society. 2 (5): 403–417. ISSN 1742-5689. PMC 1618502
 . PMID 16849201. doi:10.1098/rsif.2005.0062.
. PMID 16849201. doi:10.1098/rsif.2005.0062. - ↑ Smits, Anke M.; van Vliet, Patrick; Hassink, Rutger J.; Goumans, Marie-José; Doevendans, Pieter A. (2005-03-01). "The role of stem cells in cardiac regeneration". Journal of Cellular and Molecular Medicine. 9 (1): 25–36. ISSN 1582-1838. PMID 15784162. doi:10.1111/j.1582-4934.2005.tb00334.x.
- ↑ Bredenkamp, Nicholas; Nowell, Craig S.; Blackburn, C. Clare (2014-04-01). "Regeneration of the aged thymus by a single transcription factor". Development (Cambridge, England). 141 (8): 1627–1637. ISSN 1477-9129. PMC 3978836
 . PMID 24715454. doi:10.1242/dev.103614.
. PMID 24715454. doi:10.1242/dev.103614. - ↑ Raya-Rivera, Atlántida M.; Esquiliano, Diego; Fierro-Pastrana, Reyna; López-Bayghen, Esther; Valencia, Pedro; Ordorica-Flores, Ricardo; Soker, Shay; Yoo, James J.; Atala, Anthony (2014-07-26). "Tissue-engineered autologous vaginal organs in patients: a pilot cohort study". Lancet (London, England). 384 (9940): 329–336. ISSN 1474-547X. PMID 24726478. doi:10.1016/S0140-6736(14)60542-0.
- ↑ Auger, F. A.; Lacroix, D.; Germain, L. (2009-01-01). "Skin substitutes and wound healing". Skin Pharmacology and Physiology. 22 (2): 94–102. ISSN 1660-5535. PMID 19188757. doi:10.1159/000178868.
- ↑ "Scars - Treatment". NHS Choices. 4 September 2014. Retrieved 25 September 2016.
- ↑ "Imagine A World Without Scars" (PDF). The Scar Free Foundation. 19 July 2016. Retrieved 25 September 2016.
- ↑ Brown, B. C.; McKenna, S. P.; Siddhi, K.; McGrouther, D. A.; Bayat, A. (2008-09-01). "The hidden cost of skin scars: quality of life after skin scarring". Journal of plastic, reconstructive & aesthetic surgery: JPRAS. 61 (9): 1049–1058. ISSN 1878-0539. PMID 18617450. doi:10.1016/j.bjps.2008.03.020.
Further reading
- Kishi, K., Okabe, K., Shimizu, R., Kubota, Y. 2012. Fetal Skin Posses the Ability to Regenerate Completely: Complete Regeneration of Skin. The Keio Journal of Medicine. 61(4): 101–108. (PMID 23324304)