Salt bridge
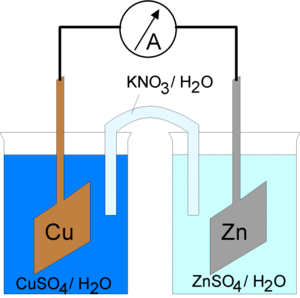
A salt bridge, in electrochemistry, is a laboratory device used to connect the oxidation and reduction half-cells of a galvanic cell (voltaic cell), a type of electrochemical cell. It maintains electrical neutrality within the internal circuit, preventing the cell from rapidly running its reaction to equilibrium. If no salt bridge were present, the solution in one half cell would accumulate negative charge and the solution in the other half cell would accumulate positive charge as the reaction proceeded, quickly preventing further reaction, and hence production of electricity.[1]
Salt bridges usually come in two types: glass tube and filter paper.
Glass tube bridges
One type of salt bridge consists of a U-shaped glass tube filled with a relatively inert electrolyte; usually potassium chloride or sodium chloride is used, although the diagram here illustrates the use of a potassium nitrate solution. The electrolyte is so chosen that
- it does not react with any of the chemicals used in the cell
- the anion and cation have similar conductivity, and hence similar migratory speed.
The electrolyte is often gelified with agar-agar to help prevent the intermixing of fluids which might otherwise occur.
The conductivity of a glass tube bridge depends mostly on the concentration of the electrolyte solution. At concentrations below saturation, an increase in concentration increases conductivity. Beyond-saturation electrolyte content and narrow tube diameter may both lower conductivity.
Filter paper bridges
The other type of salt bridge consists of a filter paper, also soaked with a relatively inert electrolyte, usually potassium chloride or sodium chloride because they are chemically inert. No gelification agent is required as the filter paper provides a solid medium for conduction.
Conductivity of this kind of salt bridge depends on a number of factors: the concentration of the electrolyte solution, the texture of the filter paper and the absorbing ability of the filter paper. Generally, smoother texture and higher absorbency equates to higher conductivity.
A porous disk or other porous barrier between the two half-cells may be used instead of a salt bridge; however, they basically serve the same purpose.
See also
References
- ↑ Hogendoorn, Bob (2010). Heinemann Chemistry Enhanced (2). Melbourne, Australia: Pearson Australia. p. 416. ISBN 9781442537552.
