Citronellal
 | |
-Citronellal_3D_ball.png) (+)-Citronellal | |
-Citronellal_3D_ball.png) (-)-Citronellal | |
| Names | |
|---|---|
| IUPAC name
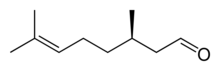
3,7-dimethyloct-6-en-1-al | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.070 |
| EC Number | 203-376-6 |
| KEGG | |
| PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C10H18O | |
| Molar mass | 154.25 g/mol |
| Density | 0.855 g/cm3 |
| Boiling point | 201 to 207 °C (394 to 405 °F; 474 to 480 K) |
| Related compounds | |
| Related alkenals |
Citral |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Citronellal or rhodinal or 3,7-dimethyloct-6-en-1-al (C10H18O) is a monoterpenoid, the main component in the mixture of terpenoid chemical compounds that give citronella oil its distinctive lemon scent.
Citronellal is a major isolate in distilled oils from the plants Cymbopogon,[2] lemon-scented gum, and lemon-scented teatree. The (–)-(S)-enantiomer of citronellal makes up to 80% of the leaf oil from kaffir lime leaves and is the compound responsible for its characteristic aroma.
Citronellal has insect repellent properties, and research shows high repellent effectiveness against mosquitoes.[3] Research shows that citronellal has strong antifungal qualities.[4]
Compendial status
See also
References
- ↑ Citronellal, The Merck Index, 12th Edition
- ↑ Mahalwal, Vijender S.; Ali, Mohd. (2003). "Volatile constituents ofCymbopogon nardus (Linn.) Rendle". Flavour and Fragrance Journal. 18: 73. doi:10.1002/ffj.1144.
- ↑ Jeong-Kyu KIM; Chang-Soo KANG; Jong-Kwon LEE; Young-Ran KIM; Hye-Yun HAN; Hwa Kyung YUN (2005). "Evaluation of Repellency Effect of Two Natural Aroma Mosquito Repellent Compounds, Citronella and Citronellal". Entomological Research. 35 (2): 117–120. doi:10.1111/j.1748-5967.2005.tb00146.x.
- ↑ Kazuhiko NAKAHARA, Najeeb S. ALZOREKY1, Tadashi YOSHIHASHI, Huong T. T. NGUYEN and Gassinee TRAKOONTIVAKORN (2003). "Chemical Composition and Antifungal Activity of Essential Oil from Cymbopogon nardus (Citronella Grass)". JARQ. 37 (4).
- ↑ The British Pharmacopoeia Secretariat (2009). "Index, BP 2009" (PDF). Archived from the original (PDF) on 11 April 2009. Retrieved 31 March 2010.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.