Rhenium pentachloride
 | |
| Names | |
|---|---|
| IUPAC name
Rhenium pentachloride | |
| Other names
Rhenium(V) chloride, Rhenium chloride, pentachlororhenium | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.660 |
| EC Number | 237-042-6 |
| PubChem CID |
|
| |
| |
| Properties | |
| ReCl5 | |
| Molar mass | 363.471 g/mol |
| Appearance | red-brown |
| Density | 4.9 g/cm3, solid |
| Melting point | 220 °C (428 °F; 493 K) |
| Boiling point | N/A |
| Will react to decompose and release HCl (g) | |
| +1225.0·10−6 cm3/mol | |
| Structure | |
| Monoclinic, mP48; a = 0.924 nm, b = 1.154 nm, c = 1.203 nm, α = 90°, β = 109.1°, γ = 90° [1] | |
| P21/c, No. 14 | |
| Octahedral | |
| Hazards | |
| Main hazards | releases HCl upon hydrolysis |
| Safety data sheet | MSDS |
| R/S statement (outdated) | R: 36, 37, 38 |
| NFPA 704 | |
| Related compounds | |
| Other anions |
Rhenium hexafluoride |
| Related compounds |
Trirhenium nonachloride, rhenium tetrachloride, rhenium hexachloride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Rhenium pentachloride is an inorganic compound of chlorine and rhenium. The compound has the formula Re2Cl10 but it is usually referred to as the pentachloride. It is a red-brown solid. It is the highest chloride of rhenium.
Structure and preparation
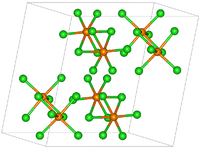
Rhenium pentachloride has a bioctahedral structure and can be formulated as Cl4Re(μ-Cl)2ReCl4. The Re-Re distance is 3.74 Å.[1] The motif is similar to that seen for tantalum pentachloride.
This compound was first prepared in 1933,[2] a few years after the discovery of rhenium. The preparation involves chlorination of rhenium at temperatures up to 900 °C.[3] The material can be purified by sublimation.
ReCl5 is one of the most oxidized binary chlorides of Re. With a d2 configuration, it could conceivably be further chlorinated, and indeed ReCl6 has been prepared, albeit indirectly from rhenium hexafluoride.[4] Rhenium heptafluoride is known but not the heptachloride.[5]
Uses and reactions
It degrades in air to a brown liquid.[6]
Although rhenium pentachloride has no commercial applications, it is of historic significance as one of the early catalysts for olefin metathesis.[7] Reduction gives trirhenium nonachloride.
Oxygenation affords the Re(VII) oxychloride:[8]
- ReCl5 + 3 Cl2O → ReO3Cl + 5 Cl2
References
- 1 2 Mucker, K. F.; Smith, G. S.; Johnson, Q. (1968). "The crystal structure of ReCl5". Acta Crystallographica Section B. 24 (6): 874. doi:10.1107/S0567740868003316.
- ↑ Geilmann, Wilhelm; Wrigge, Friedrich W.; Biltz, Wilhelm. (1933). "Rheniumpentachlorid". Z. Anorg. Allg. Chem. (in German). 214 (3): 244. doi:10.1002/zaac.19332140304.
- ↑ Roger Lincoln, Geoffrey Wilkinson "Rhenium Pentachloride and Volatile Metal Chlorides by Direct Chlorination Using a Vertical-Tube Reactor" Inorganic Syntheses, 1980, Volume 20, Pages 41–43. doi:10.1002/9780470132517.ch11.
- ↑ Tamadon, Farhad; Seppelt, Konrad (2013). "The Elusive Halides VCl5, MoCl6, and ReCl6". Angew. Chem. Int. Ed. 52: 767–769. doi:10.1002/anie.201207552.
- ↑ Stuart A. Macgregor and Klaus H. Moock "Stabilization of High Oxidation States in Transition Metals. 2.1 WCl6 Oxidizes [WF6]-, but Would PtCl6 Oxidize [PtF6]-? An Electrochemical and Computational Study of 5d Transition Metal Halides: [MF6]z versus [MCl6]z (M = Ta to Pt; z = 0, 1−, 2−)" pp 3284–3292. doi:10.1021/ic9605736
- ↑ Edwards, D. A.; Ward, R. T. (1970). "Some reactions of rhenium(V) chloride". Journal of the Chemical Society a Inorganic Physical Theoretical: 1617. doi:10.1039/J19700001617.
- ↑ Ring-opening polymerization of endo and exo-dicyclopentadiene and their 7,8-dihydro derivatives, Hamilton, J.G.; Ivin, K.J.; Rooney, J.J. Journal of Molecular Catalysis 1986 , 36, 115.
- ↑ Housecroft, C. E.; Sharpe, A. G. (2004). Inorganic Chemistry (2nd ed.). Prentice Hall. ISBN 978-0130399137.
