Ratite
| Ratites Temporal range: Paleocene-Holocene 56–0 Ma Possible Late Cretaceous record | |
|---|---|
 | |
| Southern cassowary (Casuarius casuarius) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Aves |
| Infraclass: | Palaeognathae Pycraft, 1900[1] |
| Orders | |
|
Struthioniformes ostrich | |
| Synonyms | |
|
Grallae Linnaeus, 1760[2] | |
A ratite is any of a diverse group of large, flightless birds of the infraclass Palaeognathae. The systematics involved have been in flux. Some sources state that ratites include all the flightless birds of the Palaeognathae;[4] previously, all these birds had been assigned to the order Struthioniformes, which is more recently regarded as containing only the ostrich.[1][5] The modern bird superorder Palaeognathae consists of ratites and flighted Neotropic tinamous (compare to Neognathae).[6] Unlike other flightless birds, the ratites have no keel on their sternum – hence the name from the Latin ratis (for raft). Without this to anchor their wing muscles, they could not fly even if they were to develop suitable wings. Recent research has indicated that ratites are a paraphyletic group; tinamous fall within them, and are the sister group of the extinct moa.[6][7][8][9] This implies that flightlessness is a trait that evolved independently multiple times in different ratite lineages.[8]
Most parts of the former supercontinent Gondwana have ratites, or did have until the fairly recent past.[10][11] So did Europe in the Paleocene and Eocene, from where the first flightless paleognaths are known.[12] Ostriches were present in Asia as recently as the Holocene, although the genus is thought to have originated in Africa.[13] However, the ostrich order may have evolved in Eurasia.[13] A recent study posits a Laurasian origin for the clade.[14]
Species
Living forms
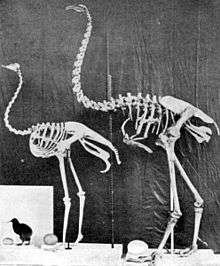
The African ostrich is the largest living ratite. A large member of this species can be nearly 2.8 metres (9.2 ft) tall, weigh as much as 156 kilograms (344 lb),[15] and can outrun a horse.
Of the living species, the Australian emu is next in height, reaching up to 1.9 metres (6.2 ft) tall and about 50 kilograms (110 lb).[15] Like the ostrich, it is a fast-running, powerful bird of the open plains and woodlands.
Also native to Australia and the islands to the north are the three species of cassowary. Shorter than an emu, but heavier and solidly built, cassowaries prefer thickly vegetated tropical forest. They can be very dangerous when surprised or cornered because of their razor sharp talons. In New Guinea, cassowary eggs are brought back to villages and the chicks raised for eating as a much-prized delicacy, despite (or perhaps because of) the risk they pose to life and limb. They reach up to 1.7 metres (5.6 ft) tall and weigh as much as 59 kilograms (130 lb)[15]
South America has two species of rhea, large fast-running birds of the Pampas. The larger American rhea grows to about 1.4 metres (4.6 ft) tall and usually weighs 15 to 40 kilograms (33–88 lb).[15]
The smallest ratites are the five species of kiwi from New Zealand. Kiwi are chicken-sized, shy, and nocturnal. They nest in deep burrows and use a highly developed sense of smell to find small insects and grubs in the soil. Kiwi are notable for laying eggs that are very large in relation to their body size. A kiwi egg may equal 15 to 20 percent of the body mass of a female kiwi. The smallest species of kiwi is the little spotted kiwi, at 0.9 to 1.9 kilograms (2.0–4.2 lb) and 35 to 45 centimetres (14–18 in).[15]
Holocene extinct forms
At least nine species of moa lived in New Zealand before the arrival of humans, ranging from turkey-sized to the giant moa Dinornis giganteus with a height of 3.7 metres (12 ft) and weighing about 230 kilograms (510 lb).[15] They became extinct by A.D. 1400 due to hunting by Māori settlers, who arrived around A.D. 1280.
Aepyornis maximus, the "elephant bird" of Madagascar, was the heaviest bird ever known. Although shorter than the tallest moa, a large A. maximus could weigh over 400 kilograms (880 lb) and stand up to 3 metres (9.8 ft) tall.[15] Accompanying it were three other species of Aepyornis as well as three species of the smaller genus Mullerornis. All these species went into decline following the arrival of humans on Madagascar around 2000 years ago, and were gone by the 17th or 18th century if not earlier.
Evolution and classification
The earliest known ratite fossils date to the Paleocene epoch about 56 million years ago (e.g. Diogenornis, a possible early relative of the rhea).[16] However, more primitive paleognaths are known from several million years earlier,[17] and the classification and membership of the Ratitae itself is uncertain. Some of the earliest ratites occur in Europe, a place frequently overlooked in the study of these birds' paleogeography in favour of gondwannan vicariance narratives.[12]
There are two taxonomic approaches to ratite classification: the one applied here combines the groups as families in the order Struthioniformes, while the other supposes that the lineages evolved mostly independently and thus elevates the families to order rank (e.g. Rheiformes, Casuariformes etc.).
Some studies based on morphology, immunology and DNA sequencing had indicated that ratites are monophyletic.[10] Based on the biogeographic vicariance hypothesis most notably described by Cracraft (1974), ancestral flightless paleognaths were present and widespread in Gondwana during the Late Cretaceous. As the supercontinent fragmented due to plate tectonics, they were carried by plate movements to their current positions and evolved into the species present today.[18]
However, recent analysis of genetic variation between the ratites conflicts with this. DNA analysis appears to show that the ratites diverged from one another too recently to share a common Gondwanian ancestor. Also, the Middle Eocene ratites such as Palaeotis and Remiornis from Central Europe may imply that the "out-of-Gondwana" hypothesis is wrong. Furthermore, recent analysis of twenty nuclear genes has drawn into question the monophyly of the group, suggesting that the flighted tinamous cluster within the ratite lineage. The authors say the data "unequivocally places tinamous within ratites".[19] A later study using forty novel nuclear loci confirmed the aforementioned conclusion.[20]
A comparative study of the full mitochondrial DNA sequences of living ratites plus two moas places moas in the basal position, followed by rheas, followed by ostriches, followed by kiwis, with emus and cassowaries being closest relatives.[10] Another study has reversed the relative positions of moas and rheas, and indicated that elephant birds are not close relatives of ostriches or other ratites,[11] while a study of nuclear genes shows ostriches branching first, followed by rheas and tinamous, then kiwis splitting from emus and cassowaries.[19] In a recent study, elephant birds were shown to be most closely related to the New Zealand kiwi.[8]
These studies share branching dates which suggest that, while ancestral moas may have been present in New Zealand since it split off from Gondwana, the ancestors of kiwis appear to have arrived there from Australia more recently, perhaps by island-hopping or powered flight. A recent extensive morphological comparison makes South American and Australian ratites a clade, with three successively more distant sister groups consisting of ostriches, elephant birds, and a clade of New Zealand ratites.[21]
All analyses but the most recent morphological study show that rheas and extant Australo-Pacific ratites are monophyletic. The DNA data showing the ostriches branching first would match the sequence of Gondwana's plate tectonic breakup.[19] Other, but not all, aspects of ratite paleobiogeography were found to be consistent with the vicariance (plate tectonic split-up of Gondwana) hypothesis. The sister-group relationship of New Zealand ratities to other ratites proposed on morphological grounds would not be consistent with vicariance, while the proposed South American-Australian clade would be.[21]
Recent phylogenomic studies suggest that tinamous belong to this group. This would make 'ratites' paraphyletic rather than monophyletic.[19][22] Since tinamous are weak fliers, this raises interesting questions about the evolution of flightlessness in this group. While the ratites were traditionally thought of as an ancestrally flightless, monophyletic group, the branching of the tinamous within the ratite lineage suggests that ratites evolved flightlessness at least three times.[19][23] Re-evolution of flight in the tinamous would be an alternative explanation, but such a development is without precedent in avian history, while loss of flight is commonplace.[19]
| Cladogram based on Mitchell et al. (2014)[8] and Yonezawa et al. (2016)[14] | |||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||
By 2014, a mitochondrial DNA phylogeny including fossil members showed tinamous nested well within the group.[8] Ostriches were placed on the first (basal) branch, followed by rheas, then a clade consisting of moas and tinamous, followed by the final two branches, a clade of emus plus cassowaries, and one of elephant birds plus kiwis.[8] The moa-tinamou relationship is consistent with other earlier and current findings,[6][7][9] while the finding of a sister relationship between elephant birds and kiwis is new. Additional support for this relationship was obtained from morphological analysis.[8]
While the basal position of ostriches would be predicted by vicariant speciation based on continental drift, the elephant bird-kiwi relation appears to require dispersal across oceans by flight; the phylogeny as a whole suggests multiple independent parallel origins of flightlessness (at least six times, or once in each major ratite lineage) and gigantism (at least five times).[8] Gigantism in birds is normally insular; however, a ten-million-year-long window of opportunity for evolution of avian gigantism on continents may have existed following the extinction of the dinosaurs, before mammals had evolved to large size. Additionally, ratite ancestors were able to fill diurnal herbivory niches originally filled by dinosaurs.[8] However, some authorities have been skeptical of the new findings and conclusions.[24]
Kiwis and tinamous are the only palaeognath lineages not to evolve gigantism, perhaps because of competitive exclusion by giant ratites already present on New Zealand and South America when they arrived or arose.[8] The fact that New Zealand has been the only land mass to support two major lineages of flightless ratites may reflect the absence of native mammals, which allowed kiwis to occupy a mammal-like nocturnal niche.[25] However, various other landmasses such as South America and Europe bear multiple lineages of flightless ratites that evolved independently, undermining this competitive exclusion hypothesis.[26]
Most recently, studies on genetic and morphological divergence and fossil distribution show that paleognaths as a whole probably had an origin in the northern hemisphere. Early Cenozoic northern hemisphere paleognaths such as Lithornis, Pseudocrypturus, Paracathartes and Palaeotis appear to be the most basal members of the clade. The various ratite lineages were probably descended from flying ancestors that colonised the south in multiple waves, probably initially in South America, and then evolved gigantism.[14]
Loss of flight

Loss of flight allows birds to eliminate the energetic cost of maintaining flight-enabling pectoral muscle mass.[27] The basal metabolic rate of volant species is much higher than that of flightless terrestrial birds.[28] But energetic efficiency can only help explain the loss of flight when the benefits of flying are not critical to fitness. Research on flightless rails indicates the flightless condition evolved in the absence of predators.[29] This shows flight to be generally necessary for survival and dispersal in birds.[30] In apparent contradiction to this, many landmasses occupied by ratites are also inhabited by predatory mammals.[8] However, the K–Pg extinction event created a window of time with large predators absent that may have allowed the ancestors of ratites to evolve flightlessness.[6] They subsequently underwent selection for large size.[6]
Description
Ratites in general have many physical characteristics in common, which are often not shared by the family Tinamidae, or tinamous. First, the breast muscles are underdeveloped. They do not have keeled sterna. Their wishbones (furculae) are almost absent. They have a simplified wing skeletons and musculature. Their legs are stronger and do not have air chambers, except the femurs. Their tail and flight feathers have retrogressed or have become decorative plumes. They have no feather vanes, which means they do not need to oil their feathers, hence they have no preen glands. They have no separation of pterylae (feathered areas) and apteria (non-feathered areas),[31] and finally, they have palaeognathous palates.[32]
Ostriches have the greatest dimorphism, rheas show some dichromatism during the breeding season. Emus, cassowaries, and kiwis show some dimorphism, predominantly in size.
While the ratites share a lot of similarities, they also have major differences. Ostriches have only two toes, with one being much larger than the other. Cassowaries have developed long inner toenails, used defensively. Ostriches and rheas have prominent wings; although they don't use them to fly, they do use them in courtship and predator distraction.[32]
Without exception, ratite chicks are capable of swimming and even diving.
On an allometric basis, paleognaths have generally smaller brains than neognaths. Kiwis are exceptions to this trend, and possess proportionally larger brains comparable to those of parrots and songbirds, though evidence for similar advanced cognitive skills is currently lacking.[33]
Gallery of living species

 Ostrich herd (S. camelus massaicus)
Ostrich herd (S. camelus massaicus)







Behavior and ecology
Feeding and diet
Ratite chicks tend to be more omnivorous or insectivorous; similarities in adults end with feeding, as they all vary in diet and length of digestive tract, which is indicative of diet. Ostriches, with the longest tracts at 14 m (46 ft), are primarily herbivorous. Rheas' tracts are next longest at between 8–9 m (26–30 ft), and they also have caeca. They are also mainly herbivores, concentrating on broad-leafed plants. However, they will eat insects if the opportunity arises. Emus have tracts of 7 m (23 ft) length, and have a more omnivorous diet, including insects and other small animals. Cassowaries have nearly the shortest tracts at 4 m (13 ft). Finally, kiwis have the shortest tracts and eat earthworms, insects, and other similar creatures.[32]
Reproduction
Ratites are different from the flying birds in that they needed to adapt or evolve certain features to protect their young. First and foremost is the thickness of the shells of their eggs. Their young are hatched more developed than most and they can run or walk soon thereafter. Also, most ratites have communal nests, where they share the incubating duties with others. Ostriches are the only ratites where the female incubates; they share the duties, with the males incubating at night. Cassowaries and emu are polyandrous, with males incubating eggs and rearing chicks with no obvious contribution from females. Ostriches and rheas are polygynous with each male courting several females. Male rheas are responsible for building nests and incubating while ostrich males incubate only at night. Kiwis stand out as the exception with extended monogamous reproductive strategies where either the male alone or both sexes incubate a single egg.[32] Without exception, ratite chicks are capable of swimming and even diving.
Ratites and humans
Ratites and humans have had a long relationship starting with the use of the egg for water containers, jewelry, or other art medium. Male ostrich feathers were popular for hats during the 18th century, which led to hunting and sharp declines in populations. Ostrich farming grew out of this need, and humans harvested feathers, hides, eggs, and meat from the ostrich. Emu farming also became popular for similar reasons and for their emu oil. Rhea feathers are popular for dusters, and eggs and meat are used for chicken and pet feed in South America. Ratite hides are popular for leather products like shoes.[32]
United States regulation
The USDA’s Food Safety and Inspection Service (FSIS) began a voluntary, fee-for-service ratite inspection program in 1995 to help the fledgling industry improve the marketability of the meat. A provision in the FY2001 USDA appropriations act (P.L. 106-387) amended the Poultry Products Inspection Act to make federal inspection of ratite meat mandatory as of April 2001 (21 U.S.C. 451 et seq.).[34]
See also
References
- 1 2 Brands, Sheila (2008-08-14). "Systema Naturae 2000 / Classification, Order Struthioniformes". Project: The Taxonomicon. Retrieved 2009-02-04.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Gray, George Robert (1863). Catalogue of British Birds in the Collection of the British Museum (PDF). Red Lion Court, Fleet Street, London, UK: Taylor and Francis. p. 133.
- 1 2 3 4 5 6 Salvadori, Tomasso; Sharpe, R. Bowdler (1895). Catalogue of the Birds in the British Museum. XXVII. Red Lion Court Fleet Street, London UK: Taylor and Francis. p. 570.
- ↑ ITIS (2007). "Struthioniformes". Integrated Taxonomic Information System. Retrieved 13 Jun 2012.
- ↑ Harshman, John; Brown, Joseph W. (13 May 2010). "Palaeognathae". The Tree of Life Web Project.
- 1 2 3 4 5 Phillips MJ, Gibb GC, Crimp EA, Penny D (January 2010). "Tinamous and moa flock together: mitochondrial genome sequence analysis reveals independent losses of flight among ratites". Systematic Biology. 59 (1): 90–107. PMID 20525622. doi:10.1093/sysbio/syp079.
- 1 2 Allentoft, M. E.; Rawlence, N. J. (2012-01-20). "Moa's Ark or volant ghosts of Gondwana? Insights from nineteen years of ancient DNA research on the extinct moa (Aves: Dinornithiformes) of New Zealand". Annals of Anatomy - Anatomischer Anzeiger. 194: 36–51. doi:10.1016/j.aanat.2011.04.002.
- 1 2 3 4 5 6 7 8 9 10 11 Mitchell, K. J.; Llamas, B.; Soubrier, J.; Rawlence, N. J.; Worthy, T. H.; Wood, J.; Lee, M. S. Y.; Cooper, A. (23 May 2014). "Ancient DNA reveals elephant birds and kiwi are sister taxa and clarifies ratite bird evolution". Science. 344 (6186): 898–900. PMID 24855267. doi:10.1126/science.1251981.
- 1 2 Baker, A. J.; Haddrath, O.; McPherson, J. D.; Cloutier, A. (2014). "Genomic Support for a Moa-Tinamou Clade and Adaptive Morphological Convergence in Flightless Ratites". Molecular Biology and Evolution. 31: 1686–1696. PMID 24825849. doi:10.1093/molbev/msu153.
- 1 2 3 Haddrath, O.; Baker, A. J. (2001). "Complete mitochondrial DNA genome sequences of extinct birds: ratite phylogenetics and the vicariance biogeography hypothesis". Proceedings of the Royal Society. Biological Sciences. 268 (1470): 939–945. PMC 1088691
 . PMID 11370967. doi:10.1098/rspb.2001.1587.
. PMID 11370967. doi:10.1098/rspb.2001.1587. - 1 2 Cooper, A.; Lalueza-Fox, C.; Anderson, S.; Rambaut, A.; Austin, J.; Ward, R. (2001-02-08). "Complete Mitochondrial Genome Sequences of Two Extinct Moas Clarify Ratite Evolution". Nature. 409 (6821): 704–707. PMID 11217857. doi:10.1038/35055536. Retrieved 2008-04-05.
- 1 2 Buffetaut, E.; Angst, D. (November 2014). "Stratigraphic distribution of large flightless birds in the Palaeogene of Europe and its palaeobiological and palaeogeographical implications". Earth-Science Reviews. 138: 394–408. doi:10.1016/j.earscirev.2014.07.001.
- 1 2 Hou, L.; Zhou, Z.; Zhang, F.; Wang, Z. (Aug 2005). "A Miocene ostrich fossil from Gansu Province, northwest China". Chinese Science Bulletin. 50 (16): 1808–1810. ISSN 1861-9541. doi:10.1360/982005-575.
- 1 2 3 Yonezawa, T.; Segawa, T.; Mori, H.; Campos, P. F.; Hongoh, Y.; Endo, H.; Akiyoshi, A.; Kohno, N.; Nishida, S.; Wu, J.; Jin, H.; Adachi, J.; Kishino, H.; Kurokawa, K.; Nogi, Y.; Tanabe, H.; Mukoyama, H.; Yoshida, K.; Rasoamiaramanana, A.; Yamagishi, S.; Hayashi, Y.; Yoshida, A.; Koike, H.; Akishinonomiya, F.; Willerslev, E.; Hasegawa, M. (2016-12-15). "Phylogenomics and Morphology of Extinct Paleognaths Reveal the Origin and Evolution of the Ratites". Current Biology. 27 (1): 68–77. PMID 27989673. doi:10.1016/j.cub.2016.10.029.
- 1 2 3 4 5 6 7 Davies, S.J.J.F. (2003). "Struthioniformes (Tinamous and Ratites)". In Hutchins, Michael; Jackson, Jerome A.; Bock, Walter J.; Olendorf, Donna. Grzimek's Animal Life Encyclopedia. 8 Birds I Tinamous and Ratites to Hoatzins (2 ed.). Farmington Hills, MI: Gale Group. pp. 56–105. ISBN 0787657840.
- ↑ Laurin, M.; Gussekloo, S.W.S.; Marjanovic, D.; Legendre, L.; Cubo, J. (2012). "Testing gradual and speciational models of evolution in extant taxa: the example of ratites". Journal of Evolutionary Biology. 25: 293–303. PMID 22107024. doi:10.1111/j.1420-9101.2011.02422.x.
- ↑ Leonard, L.; Dyke, G. J.; Van Tuinen, M. (October 2005). "A New Specimen of the Fossil Palaeognath Lithornis from the Lower Eocene of Denmark". American Museum Novitates. 3491: 1–11. doi:10.1206/0003-0082(2005)491[0001:ANSOTF]2.0.CO;2.
- ↑ Cracraft, J (October 1974). "Phylogeny and evolution of ratite birds". Ibis. 116: 494–521. doi:10.1111/j.1474-919X.1974.tb07648.x.
- 1 2 3 4 5 6 Harshman, J.; Braun, E. L.; Braun, M. J.; Huddleston, C. J.; Bowie, R. C. K.; Chojnowski, J. L.; Hackett, S. J.; Han, K.-L.; Kimball, R. T.; Marks, B. D.; Miglia, K. J.; Moore, W. S.; Reddy, S.; Sheldon, F. H.; Steadman, D. W.; Steppan, S. J.; Witt, C. C.; Yuri, T. (September 2008). "Phylogenomic evidence for multiple losses of flight in ratite birds". Proceedings of the National Academy of Sciences. 105 (36): 13462–13467. PMC 2533212
 . PMID 18765814. doi:10.1073/pnas.0803242105.
. PMID 18765814. doi:10.1073/pnas.0803242105. - ↑ Smith, J. V.; Braun, E. L.; Kimball, R. T. (January 2012). "Ratite Nonmonophyly: Independent Evidence from 40 Novel Loci". Systematic Biology. 62 (1): 35–49. PMID 22831877. doi:10.1093/sysbio/sys067.
- 1 2 Bourdon, Estelle; De Ricqles, Armand; Cubo, Jorge (2009). "A new Transantarctic relationship: morphological evidence for a Rheidae–Dromaiidae–Casuariidae clade (Aves, Palaeognathae, Ratitae)". Zoological Journal of the Linnean Society. 156 (3): 641–663. doi:10.1111/j.1096-3642.2008.00509.x.
- ↑ Hackett, Shannon J.; et al. (2008-06-27). "A Phylogenomic Study of Birds Reveals Their Evolutionary History". Science. 320 (5884): 1763–1768. PMID 18583609. doi:10.1126/science.1157704. Retrieved 2008-10-18.
- ↑ Holmes, Bob (2008-06-26). "Bird evolutionary tree given a shake by DNA study". New Scientist. Retrieved 2009-02-04.
- ↑ Zimmer, C. (2014-05-22). "A Theory on How Flightless Birds Spread Across the World: They Flew There". New York Times. Archived from the original on 2014-05-23. Retrieved 2014-05-24.
- ↑ Le Duc, D.; Renaud, G.; Krishnan, A.; Almén, M. S.; Huynen, L.; Prohaska, S. J.; Ongyerth, M.; Bitarello, B. D.; Schiöth, H. B.; Hofreiter, M.; Stadler, P. F.; Prüfer, K.; Lambert, D.; Kelso, J.; Schöneberg, T. (23 July 2015). "Kiwi genome provides insights into evolution of a nocturnal lifestyle". Genome Biology. 16 (1): 147–162. PMC 4511969
 . PMID 26201466. doi:10.1186/s13059-015-0711-4.
. PMID 26201466. doi:10.1186/s13059-015-0711-4. - ↑ Agnolin, F. L. (2016-07-05). "Unexpected diversity of ratites (Aves, Palaeognathae) in the early Cenozoic of South America: palaeobiogeographical implications". Alcheringa: An Australasian Journal of Palaeontology: 1–11. doi:10.1080/03115518.2016.1184898.
- ↑ McNab, B. K. (October 1994). "Energy Conservation and the Evolution of Flightlessness in Birds" (PDF). The American Naturalist. 144 (4): 628–648. doi:10.1086/285697.
- ↑ Cubo, Arthur (May 4, 2001). "Patterns of correlated character evolution in flightless birds: a phylogenetic approach" (PDF). Evolutionary Ecology.
- ↑ McNab, B. K.; Ellis, H. I. (November 2006). "Flightless rails endemic to islands have lower energy expenditures and clutch sizes than flighted rails on islands and continents". Comp Biochem Physiol A Mol Integr Physiol. 145 (3): 628–648. PMID 16632395. doi:10.1016/j.cbpa.2006.02.025.
- ↑ Diamond, J. (July 1991). "A New Species of Rail from the Solomon-Islands and Convergent Evolution of Insular Flightlessness". Auk. 108 (3): 461–470. doi:10.2307/4088088.
- ↑ http://www.freedictionary.com for definitions of the two latin words
- 1 2 3 4 5 Bruning, D. F. (2003). "Rheas". In Hutchins, Michael. Grzimek's Animal Life Encyclopedia. 8 Birds I Tinamous and Ratites to Hoatzins (2 ed.). Farmington Hills, MI: Gale Group. pp. 53–55. ISBN 0-7876-5784-0.
- ↑ Corfield, J. R.; Wild, J. M.; Hauber, M. E.; Parsons, S.; Kubke, M. F. (2007-11-21). "Evolution of Brain Size in the Palaeognath Lineage, with an Emphasis on New Zealand Ratites". Brain, Behavior and Evolution. 71 (2): 87–99. doi:10.1159/000111456.
- ↑ Womach, Jasper (2005). "Agriculture: A Glossary of Terms, Programs, and Laws" (PDF). 2005. Retrieved 15 Jul 2009.
External links
| Wikimedia Commons has media related to Palaeognathae. |
| Wikisource has the text of the 1920 Encyclopedia Americana article Ratitæ. |