Radical fluorination
Radical fluorination is a type of fluorination reaction, complementary to nucleophilic and electrophilic approaches.[1] It involves the reaction of an independently generated carbon-centered radical with an atomic fluorine source and yields an organofluorine compound.
Historically, only three atomic fluorine sources were available for radical fluorination: Fluorine (F2), hypofluorites (O—F based reagents) and XeF2. Their high reactivity, and the difficult handling of F2 and the hypofluorites, limited the development of radical fluorination compared to electrophilic and nucleophilic methods.[2] The uncovering of the ability of electrophilic N—F fluorinating agents to act as atomic fluorine source[3] led to a renaissance in radical fluorination.[2]
Various methodologies have since been developed for the radical formation of C—F bonds.[1] The radical intermediates have been generated from carboxylic acids and boronic acid derivatives, by radical addition to alkenes, or C—H bond and C—C bond activations. New sources of atomic fluorine are now emerging, such as metal-fluoride complexes.
Sources of atomic fluorine
Fluorine gas
Fluorine can act both as an electrophilic and atomic source of fluorine.[4] The weak F—F bond strength (36 kcal/mol (150 kJ/mol)[5]) allows for homolytic cleavage. The reaction of F2 with organic compounds is, however, really exothermic and can lead to non-selective fluorinations and C—C cleavage, as well as explosions.[6] Only a few selective radical fluorination methods have been reported.[7][8] The use of fluorine for radical fluorination is mainly limited to perfluorination reactions.[5]
O—F reagents
The O—F bond of hypofluorites is relatively weak. For trifluoromethyl hypofluorite (CF3OF), it was estimated to be 43.5 kcal/mol (182 kJ/mol).[9] The ability of trifluoromethyl hypofluorite to transfer fluorine to alkyl radicals was notably demonstrated by reacting independently generated ethyl radicals from ethene and tritium in presence of CF3OF.[10] The high reactivity of hypofluorites has limited their application to selective radical fluorination. They can, however, be used as radical initiators for polymerization.[11]
XeF2
XeF2 has mainly been used for radical fluorination in radical decarboxylative fluorination reactions.[12] In this Hunsdiecker-type reaction, xenon difluoride is used to generate the radical intermediate, as well as the fluorine transfer source.[13]
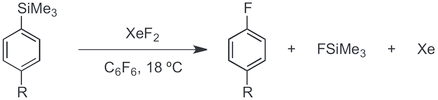
XeF2 can also be used to generate aryl radicals from aryl silanes, and act as atomic fluorine source to furnish aryl fluorides.[14]
N—F reagents
Selectfluor and N-fluorobenzenesulfonimide (NFSI) are traditionally used as electrophilic sources of fluorine, but their ability to transfer fluorine to alkyl radical has recently been demonstrated.[3] They are now commonly used as fluorine transfer agents to alkyl radicals.[1]
Others
Examples of radical fluorination using BrF3[15] and fluorinated solvents[16] have been reported. Recent examples in radical fluorination suggest that in-situ generated metal-fluoride complexes can also act as fluorine transfer agent to alkyl radicals.
Radical fluorination methodologies
Decarboxylative fluorination
Thermolysis of t-butyl peresters has been used to generate alkyl radicals in presence of NFSI and Selectfluor.[3] The radicals intermediates were efficiently fluorinated, demonstrating the ability of the two electrophilic fluorinating agents to transfer fluorine to alkyl radicals.

Carboxylic acids can be used as radical precursors in radical fluorination methods. Metal catalysts such as silver[17] and manganese[18] have been used to induce the fluorodecarboxylation. The fluorodecarboxylation of carboxylic acids can also be triggered using photoredox catalysis.[19][20] More specifically, phenoxyacetic acid derivatives have been shown to undergo fluorodecarboxylation when directly exposed to UV-light irradiation[21] or via the use of a photosensitizer.[22]

Radical fluorination of alkenes
Alkyl radicals generated from radical additions to alkenes have also been fluorinated. Hydrides[23] and nitrogen-,[24] carbon-,[25] and phosphorus-centered[26] radicals have been employed, yielding a wide range of fluorinated, difunctionalized compounds.
Fluorination of boronic acid derivatives
Alkyl fluorides have been synthesized via radicals generated from boronic acid derivatives using silver.[27]

C(sp3)—H fluorination
One major advantage of radical fluorination is that it allows the direct fluorination of remote C—H bonds. Metal catalysts such as Mn,[28] Cu[29] or W[30] have been used to promote the reaction. Metal-free C(sp3)—H fluorinations rely on the use of radical initiators (Et3B,[31] persulfates[32] or N-oxyl radicals[33]) or organic photocatalysts.[33]
Some methods have also been developed to selectively fluorinate benzylic C—H bonds.[34]
C—C bonds activation
Cyclobutanols and cyclopropanols have been used as radical precursors for the synthesis of β- or γ-fluoroketones. The strained rings undergo C—C bond cleavage in presence of a silver[35][36] or an iron catalyst[36] or when exposed to UV-light in presence of a photosensitizer.[37]

Potential application
One potential application of radical fluorination is for efficiently accessing novel moieties to serve as building blocks in medicinal chemistry.[38] Derivatives of propellane with reactive functional groups, such as the hydrochloride salt of 3-fluorobicyclo[1.1.1]pentan-1-amine, are accessible by this approach.[38]
References
- 1 2 3 Paquin, Jean-François; Sammis, Glenn; Chatalova-Sazepin, Claire; Hemelaere, Rémy (2015-08-03). "Recent Advances in Radical Fluorination". Synthesis. 47 (17): 2554–2569. doi:10.1055/s-0034-1378824.
- 1 2 Sibi, Mukund P.; Landais, Yannick (2013). "C sp 3—F Bond Formation: A Free-Radical Approach". Angewandte Chemie International Edition. 52 (13): 3570–3572. doi:10.1002/anie.201209583.
- 1 2 3 Rueda-Becerril, Montserrat; Chatalova Sazepin, Claire; Leung, Joe C. T.; Okbinoglu, Tulin; Kennepohl, Pierre; Paquin, Jean-François; Sammis, Glenn M. (2012-03-07). "Fluorine Transfer to Alkyl Radicals". Journal of the American Chemical Society. 134 (9): 4026–4029. ISSN 0002-7863. doi:10.1021/ja211679v.
- ↑ Bigelow, Lucius A. (1947-02-01). "The Action of Elementary Fluorine upon Organic Compounds.". Chemical Reviews. 40 (1): 51–115. ISSN 0009-2665. doi:10.1021/cr60125a004.
- 1 2 Hutchinson, John; Sandford, Graham (1997-01-01). S, Prof Richard D. Chambers F. R., ed. Elemental Fluorine in Organic Chemistry. Topics in Current Chemistry. Springer Berlin Heidelberg. pp. 1–43. ISBN 978-3-540-63170-5. doi:10.1007/3-540-69197-9_1.
- ↑ Simons, J. H.; Block, L. P. (1939-10-01). "Fluorocarbons. The Reaction of Fluorine with Carbon". Journal of the American Chemical Society. 61 (10): 2962–2966. ISSN 0002-7863. doi:10.1021/ja01265a111.
- ↑ Grakauskas, Vytautas (1969-08-01). "Aqueous fluorination of carboxylic acid salts". The Journal of Organic Chemistry. 34 (8): 2446–2450. ISSN 0022-3263. doi:10.1021/jo01260a040.
- ↑ Bockemüller, Wilhelm (1933-01-01). "Versuche zur Fluorierung organischer Verbindungen. III. Über die Einwirkung von Fluor auf organische Verbindungen". Justus Liebigs Annalen der Chemie. 506 (1): 20–59. ISSN 1099-0690. doi:10.1002/jlac.19335060103.
- ↑ Czarnowski, J.; Castellano, E.; Schumacher, H. J. "The energy of the O?F bond in trifluoromethyl hypofluorite". Chemical Communications (London) (20): 1255. doi:10.1039/c19680001255.
- ↑ Wang, Nunyii; Rowland, F. S. (1985-11-01). "Trifluoromethyl hypofluorite: a fluorine-donating radical scavenger". The Journal of Physical Chemistry. 89 (24): 5154–5155. ISSN 0022-3654. doi:10.1021/j100270a006.
- ↑ Francesco, Venturini; Sansotera, Maurizio; Navarrini, Walter (2013-11-01). "Recent developments in the chemistry of organic perfluoro hypofluorites". Journal of Fluorine Chemistry. 2013 ACS Fluorine Award Issue: Professor Iwao Ojima. 155: 2–20. doi:10.1016/j.jfluchem.2013.07.005.
- ↑ Tius, Marcus A. (1995-06-12). "Xenon difluoride in synthesis". Tetrahedron. 51 (24): 6605–6634. doi:10.1016/0040-4020(95)00362-C.
- ↑ Patrick, Timothy B.; Darling, Diana L. (1986-08-01). "Fluorination of activated aromatic systems with cesium fluoroxysulfate". The Journal of Organic Chemistry. 51 (16): 3242–3244. ISSN 0022-3263. doi:10.1021/jo00366a044.
- ↑ Lothian, Aileen P.; Ramsden, Christopher A. (1993-01-01). "Rapid Fluorodesilylation of Aryltrimethylsilanes Using Xenon Difuoride: An Efficient New Route to Aromatic Fluorides". Synlett. 1993 (10): 753–755. doi:10.1055/s-1993-22596.
- ↑ Sasson, Revital; Rozen, Shlomo (2005-01-31). "Constructing the CF3 group; unique trifluorodecarboxylation induced by BrF3". Tetrahedron. 61 (5): 1083–1086. doi:10.1016/j.tet.2004.11.063.
- ↑ Yamada, Shigeyuki; Gavryushin, Andrei; Knochel, Paul (2010). "Convenient Electrophilic Fluorination of Functionalized Aryl and Heteroaryl Magnesium Reagents". Angewandte Chemie International Edition. 49 (12): 2215–2218. PMID 20162637. doi:10.1002/anie.200905052.
- ↑ Yin, Feng; Wang, Zhentao; Li, Zhaodong; Li, Chaozhong (2012-06-27). "Silver-Catalyzed Decarboxylative Fluorination of Aliphatic Carboxylic Acids in Aqueous Solution". Journal of the American Chemical Society. 134 (25): 10401–10404. ISSN 0002-7863. PMID 22694301. doi:10.1021/ja3048255.
- ↑ Huang, Xiongyi; Liu, Wei; Hooker, Jacob M.; Groves, John T. (2015-04-20). "Targeted Fluorination with the Fluoride Ion by Manganese-Catalyzed Decarboxylation". Angewandte Chemie International Edition. 54 (17): 5241–5245. ISSN 1521-3773. doi:10.1002/anie.201500399.
- ↑ Rueda-Becerril, Montserrat; Mahé, Olivier; Drouin, Myriam; Majewski, Marek B.; West, Julian G.; Wolf, Michael O.; Sammis, Glenn M.; Paquin, Jean-François (2014-01-30). "Direct C–F Bond Formation Using Photoredox Catalysis". Journal of the American Chemical Society. 136 (6): 2637–2641. PMID 24437369. doi:10.1021/ja412083f.
- ↑ Ventre, Sandrine; Petronijevic, Filip R.; MacMillan, David W. C. (2015-04-27). "Decarboxylative Fluorination of Aliphatic Carboxylic Acids via Photoredox Catalysis". Journal of the American Chemical Society. 137 (17): 5654–5657. PMC 4862610
 . PMID 25881929. doi:10.1021/jacs.5b02244.
. PMID 25881929. doi:10.1021/jacs.5b02244. - ↑ Leung, Joe C. T.; Chatalova-Sazepin, Claire; West, Julian G.; Rueda-Becerril, Montserrat; Paquin, Jean-François; Sammis, Glenn M. (2012-10-22). "Photo-fluorodecarboxylation of 2-Aryloxy and 2-Aryl Carboxylic Acids". Angewandte Chemie International Edition. 51 (43): 10804–10807. ISSN 1521-3773. PMID 23023887. doi:10.1002/anie.201206352.
- ↑ Leung, Joe C. T.; Sammis, Glenn M. (2015-04-01). "Radical Decarboxylative Fluorination of Aryloxyacetic Acids Using N-Fluorobenzenesulfonimide and a Photosensitizer". European Journal of Organic Chemistry. 2015 (10): 2197–2204. ISSN 1099-0690. doi:10.1002/ejoc.201500038.
- ↑ Barker, Timothy J.; Boger, Dale L. (2012-08-07). "Fe(III)/NaBH 4 -Mediated Free Radical Hydrofluorination of Unactivated Alkenes". Journal of the American Chemical Society. 134 (33): 13588–13591. PMC 3425717
 . PMID 22860624. doi:10.1021/ja3063716.
. PMID 22860624. doi:10.1021/ja3063716. - ↑ Li, Zhaodong; Zhang, Chengwei; Zhu, Lin; Liu, Chao; Li, Chaozhong. "Transition-metal-free, room-temperature radical azidofluorination of unactivated alkenes in aqueous solution". Org. Chem. Front. 1 (1): 100–104. doi:10.1039/c3qo00037k.
- ↑ Kindt, Stephanie; Heinrich, Markus R. (2014-11-17). "Intermolecular Radical Carbofluorination of Non-activated Alkenes". Chemistry: A European Journal. 20 (47): 15344–15348. ISSN 1521-3765. doi:10.1002/chem.201405229.
- ↑ Zhang, Chengwei; Li, Zhaodong; Zhu, Lin; Yu, Limei; Wang, Zhentao; Li, Chaozhong (2013-09-13). "Silver-Catalyzed Radical Phosphonofluorination of Unactivated Alkenes". Journal of the American Chemical Society. 135 (38): 14082–14085. PMID 24025164. doi:10.1021/ja408031s.
- ↑ Li, Zhaodong; Wang, Zhentao; Zhu, Lin; Tan, Xinqiang; Li, Chaozhong (2014-11-06). "Silver-Catalyzed Radical Fluorination of Alkylboronates in Aqueous Solution". Journal of the American Chemical Society. 136 (46): 16439–16443. PMID 25350556. doi:10.1021/ja509548z.
- ↑ Liu, Wei; Huang, Xiongyi; Cheng, Mu-Jeng; Nielsen, Robert J.; Goddard, William A.; Groves, John T. (2012-09-14). "Oxidative Aliphatic C-H Fluorination with Fluoride Ion Catalyzed by a Manganese Porphyrin". Science. 337 (6100): 1322–1325. ISSN 0036-8075. PMID 22984066. doi:10.1126/science.1222327.
- ↑ Bloom, Steven; Pitts, Cody Ross; Miller, David Curtin; Haselton, Nathan; Holl, Maxwell Gargiulo; Urheim, Ellen; Lectka, Thomas (2012-10-15). "A Polycomponent Metal-Catalyzed Aliphatic, Allylic, and Benzylic Fluorination". Angewandte Chemie International Edition. 51 (42): 10580–10583. ISSN 1521-3773. PMID 22976771. doi:10.1002/anie.201203642.
- ↑ Halperin, Shira D.; Fan, Hope; Chang, Stanley; Martin, Rainer E.; Britton, Robert (2014). "A Convenient Photocatalytic Fluorination of Unactivated C—H Bonds". Angewandte Chemie International Edition. 53 (18): 4690–4693. PMID 24668727. doi:10.1002/anie.201400420.
- ↑ Pitts, Cody Ross; Ling, Bill; Woltornist, Ryan; Liu, Ran; Lectka, Thomas (2014-08-27). "Triethylborane-Initiated Radical Chain Fluorination: A Synthetic Method Derived from Mechanistic Insight". The Journal of Organic Chemistry. 79 (18): 8895–8899. PMID 25137438. doi:10.1021/jo501520e.
- ↑ Zhang, Xiaofei; Guo, Shuo; Tang, Pingping. "Transition-metal free oxidative aliphatic C–H fluorination". Org. Chem. Front. 2 (7): 806–810. doi:10.1039/c5qo00095e.
- 1 2 Amaoka, Yuuki; Nagatomo, Masanori; Inoue, Masayuki (2013-04-19). "Metal-Free Fluorination of C(sp 3 )–H Bonds Using a Catalytic N -Oxyl Radical". Organic Letters. 15 (9): 2160–2163. PMID 23600550. doi:10.1021/ol4006757.
- ↑ Koperniku, Ana; Liu, Hongqiang; Hurley, Paul B. (2016-01-15). "Mono- and Difluorination of Benzylic Carbon Atoms". European Journal of Organic Chemistry. 2016: 871–886. ISSN 1099-0690. doi:10.1002/ejoc.201501329.
- ↑ Ishida, Naoki; Okumura, Shintaro; Nakanishi, Yuuta; Murakami, Masahiro (2015-01-01). "Ring-opening Fluorination of Cyclobutanols and Cyclopropanols Catalyzed by Silver". Chemistry Letters. 44 (6): 821–823. doi:10.1246/cl.150138.
- 1 2 Ren, Shichao; Feng, Chao; Loh, Teck-Peng. "Iron- or silver-catalyzed oxidative fluorination of cyclopropanols for the synthesis of β-fluoroketones". Org. Biomol. Chem. 13 (18): 5105–5109. doi:10.1039/c5ob00632e.
- ↑ Bloom, Steven; Bume, Desta Doro; Pitts, Cody Ross; Lectka, Thomas (2015-05-26). "Site-Selective Approach to β-Fluorination: Photocatalyzed Ring Opening of Cyclopropanols". Chemistry: A European Journal. 21 (22): 8060–8063. ISSN 1521-3765. doi:10.1002/chem.201501081.
- 1 2 Goha, Y. L.; Adsool, V. A. (2015). "Radical fluorination powered expedient synthesis of 3-fluorobicyclo[1.1.1]pentan-1-amine". Org. Biomol. Chem. 13: 11597–11601. doi:10.1039/C5OB02066B.