Radialene
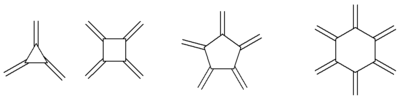
[n]Radialenes are alicyclic organic compounds containing n cross-conjugated exocyclic double bonds.[1][2][3][4] The double bonds are commonly alkene groups but those with a carbonyl (C=O) group are also called radialenes.[5] For some members the unsubstituted parent radialenes are elusive but many substituted derivatives are known.
Radialenes are related to open-chain dendralenes and also to compounds like butadiene and benzene which also consist of a ring of sp2 hybridized carbon atoms.
Radialenes are investigated in organic chemistry for their unusual properties and reactivity but have not ventured outside the laboratory. Reported uses are as experimental building blocks for novel organic conductors and ferromagnets.[6] The first radialene called hexaethylidencyclohexane was synthesised in 1961.[7]
Conformation
[3] and [4]radialenes are expected to have a planar molecular geometry with all carbon atoms in the same plane. This is verified experimentally in hexamethyl[3]radialene and cyclobutanetetraonetetrakis(hydrazone). Decamethyl[5]radialene has a twist envelope geometry with C2 symmetry while a chair conformation is calculated for [6]radialene and found experimentally for hexa-(ethylidene)cyclohexane
Due to their specific pi-electron distributions, hydrocarbons such as perylene and triphenylene are not considered radialenes. One study [6] describes a [6]radialene composed of thiophene units:[8]
This compound is reported as planar with D3h symmetry (X-ray diffraction) but not aromatic: the carbon-carbon bond lengths are unusually long (145 pm vs. 140 pm for benzene) and the calculated NICS value is close to zero.
Synthesis and properties
The parent [3],[4],[5] and [6]radialenes polymerize when in contact with oxygen.
[3]Radialenes
[3]Radialene or trimethylenecyclopropane was synthesised in 1965.[9][10][11] Reported derivatives are triquinocyclopropanes,[12][13][14] salts of trimethylenecyclopropane dianions,[15] tris(thioxanthen-9-ylidene)cyclopropane,[16] tris(fluoren-9-ylidene)cyclopropane [17] and hexakis(trimethylsilylethynyl)[3]radialene.[18] Phosphorus derivatives (based on 4,5,6-triphospha[3]radialene) have also been reported.[19][20][21][22] Phospharadialenes have been investigated as quantum efficiency improvers in solar cells [23] Hexakis[4-(diarylamino)phenyl][3]radialene derivatives have been investigated for their low oxidation potentials.[24]
[4]Radialenes
The unsubstituted [4]radialene has been prepared in an elimination reaction of cis,trans,cis-tetra(bromomethyl)cyclobutane with sodium methoxide in ethanol.[25]

Hydrogenation with platinum on carbon gives cis,cis,cis-tetramethylcyclobutane in accordance with the proposed structure. On standing in air at room temperature the compound accepts oxygen and polymerizes.
[5]Radialenes
Successful low-temperature synthesis of the parent compound [5]radialene was reported in 2015.[26]
[6]Radialenes
The parent [6]radialene is unstable and polymerises immediately on formation. It has been synthesised from 1,5,9-cyclododecatriyne, 1,3,6-tri(chloromethyl)mesitylene and tricyclobutabenzene.[27][28][29][30]
Only substituted [6]radialenes exist as stable compounds. Stable derivatives are the hexamethyl substituted,[7][31] dodecamethyl substituted [32] and hexabromo substituted [33] radialene.
A trisalkoxy-substituted radialene has also been reported,[34] the central ring adopting a non-planar twist-boat conformation:
Uses
Radialenes have been researched as a potential way to access complex synthetic molecules [35][36] and in polymer synthesis.[37][38]
References
- ↑ The Chemistry of Dienes and Polyenes, Volume 1 The Chemistry of Dienes and Polyenes Volume 121 Patai's Chemistry of Functional Groups Zvi Rappoport Ed. Wiley, 1997
- ↑ Iyoda, M., Yamakawa, J. and Rahman, M. J. (2011), Conjugated Macrocycles: Concepts and Applications. Angew. Chem. Int. Ed., 50: 10522–10553. doi:10.1002/anie.201006198
- ↑ Oligomeric and Polymeric Systems with a Cross-conjugated π-Framework Mojtaba Gholami and Rik R. Tykwinski Chemical Reviews 2006 106 (12), 4997-5027 doi:10.1021/cr0505573
- ↑ Hopf, H. and Maas, G. (1992), Preparation and Properties, Reactions, and Applications of Radialenes. Angew. Chem. Int. Ed. Engl., 31: 931–954. doi:10.1002/anie.199209313
- ↑ Effect of Overcrowding in [n]Radialenes on the Synthesis of Bis[4]radialenesMenahem Kaftory, Mark Botoshansky, Shunji Hyoda, Toshihiro Watanabe, and Fumio Toda J. Org. Chem.; 1999; 64(7) pp 2287 - 2292; (Article) doi:10.1021/jo9818
- 1 2 Planar [6]Radialenes: Structure, Synthesis, and Aromaticity of Benzotriselenophene and Benzotrithiophene Asit Patra, Yair H. Wijsboom, Linda J. W. Shimon, and Michael Bendikov Angew. Chem. Int. Ed. 2007, 46, 8814 –8818 doi:10.1002/anie.200703123
- 1 2 Hopff, H.; Wick, A. K. (1961). "Zur Kenntnis der Hexaalkylbenzole. 3. Mitteilung. Über einen neuen Kohlenwasserstoff C18H24 (Hexaäthylidencyclohexan)". Helvetica Chimica Acta. 44 (2): 380. doi:10.1002/hlca.19610440206.
- ↑ Coupling reaction reagent bis(1,5-cyclooctadiene)nickel(0). Equal amount of tetramer formed
- ↑ The Preparation and Properties of Trimethylenecyclopropane Ernest A. Dorko Journal of the American Chemical Society 1965 87 (23), 5518-5520 doi:10.1021/ja00951a067
- ↑ The molecular structure of trimethylenecyclopropane E.A. Dorko , J.L. Hencher, S.H. Bauer Tetrahedron Volume 24, Issue 6, 1968, Pages 2425–2434 doi:10.1016/S0040-4020(01)82515-1
- ↑ Bally, T. and Haselbach, E. (1978), Tris (methylidene)-cyclopropane (“[3]Radialene”). Part 2. Electronic states of the molecular cation and revised UV.-absorption spectrum of the parent neutral. HCA, 61: 754–761. doi:10.1002/hlca.19780610223
- ↑ Synthesis of a Triquinocyclopropane Robert. West and David C. Zecher Journal of the American Chemical Society 1967 89 (1), 152-153 doi:10.1021/ja00977a033
- ↑ Diarylquinocyclopropenes and triquinocyclopropanes Robert West and David C. Zecher Journal of the American Chemical Society 1970 92 (1), 155-161 doi:10.1021/ja00704a025
- ↑ Polyanthraquinocyclopropanes, dianthraquinocyclopropanone, and dianthraquinoethylene. Synthesis and properties Judith L. Benham, Robert West, and John A. T. Norman Journal of the American Chemical Society 1980 102 (15), 5047-5053 doi:10.1021/ja00535a037
- ↑ Negatively substituted trimethylenecyclopropane dianions Tadamichi Fukunaga Journal of the American Chemical Society 1976 98 (2), 610-611 doi:10.1021/ja00418a050
- ↑ Sugimoto, T., Misaki, Y., Kajita, T., Nagatomi, T., Yoshida, Z.-i. and Yamauchi, J. (1988), Tris(thioxanthen-9-ylidene)cyclopropane, and Its Radical Cation and Dication. Angew. Chem. Int. Ed. Engl., 27: 1078–1080. doi: 10.1002/anie.198810781
- ↑ Iyoda, M., Otani, H. and Oda, M. (1988), Tris(fluoren-9-ylidene)cyclopropane, a Novel [3]Radialene. Angew. Chem. Int. Ed. Engl., 27: 1080–1081. doi:10.1002/anie.198810801
- ↑ Lange, T., Gramlich, V., Amrein, W., Diederich, F., Gross, M., Boudon, C. and Gisselbrecht, J.-P. (1995), Hexakis(trimethylsilylethynyl)[3]radialene: A Carbon-Rich Chromophore with Unusual Electronic Properties. Angew. Chem. Int. Ed. Engl., 34: 805–809. doi:10.1002/anie.199508051
- ↑ Miyake, H., Sasamori, T. and Tokitoh, N. (2012), Synthesis and Properties of 4,5,6-Triphospha[3]radialene. Angew. Chem. Int. Ed., 51: 3458–3461. doi:10.1002/anie.201200374
- ↑ The 4,5,6-triphospha[3]radialene dianion: a phosphorus analogue of the deltate dianion. A NICS(0)πzz examination of their aromaticity Hideaki Miyake, Takahiro Sasamori, Judy I-Chia Wu, Paul v. R. Schleyer and Norihiro Tokitoh Chem. Commun., 2012,48, 11440-11442 doi:10.1039/C2CC35978B
- ↑ The first x-ray analysis of a phospha[3]radialene, Polyhedron Volume 11, Issue 3, 1992, Pages 385-387 Ikuko Miyahara, Atsuhiro Hayashi, Ken HirotsuMasaaki Yoshifuji, Hideki Yoshimura, Kozo Toyota doi:10.1016/S0277-5387(00)83187-3
- ↑ Photochemical (E)–(Z) Isomerization of the P=C Double Bond in Triphospha[3]radialene–[M(CO)5] (M = W, Cr) Complexes Takahiro Sasamori, Koki Hirano, Hideaki Miyake, Norihiro Tokitoh Chemistry Letters Vol. 44 (2015) No. 9 P 1240-1242 doi:10.1246/cl.150422
- ↑ Hopf, H. (2012), Phospharadialenes—A New Kid in Town. Angew. Chem. Int. Ed., 51: 11945–11947. doi:10.1002/anie.201206101
- ↑ Triarylamines on [3]Radialene Scaffold: Novel [3]Radialene-based, Multistep, Wide-range Redox Systems with Remarkably Low Oxidation Potentials Kouzou Matsumoto, Nao Yamada, Tetsuya Enomoto, Hiroyuki Kurata, Takeshi Kawase, Masaji Oda Chemistry Letters Vol. 40 (2011) No. 9 P 1033-1035 doi:10.1246/cl.2011.1033
- ↑ The Chemistry of Photodimers of Maleic and Fumaric Acid Derivatives. IV.1 Tetramethylenecyclobutane Gary W. Griffin and Laurence I. Peterson J. Am. Chem. Soc.; 1962; 84(17) pp 3398 - 3400; doi:10.1021/ja00876a033
- ↑ [5]Radialene Emily G. Mackay, Christopher G. Newton, Henry Toombs-Ruane, Erik Jan Lindeboom, Thomas Fallon, Anthony C. Willis, Michael N. Paddon-Row, and Michael S. Sherburn Journal of the American Chemical Society Article ASAP doi:10.1021/jacs.5b07445
- ↑ Barkovich, A. J.; Vollhardt, K. P. C. (1976). "1,5,9-Cyclododecatriyne. Synthesis and conversion to intermediate 1,2:3,4:5,6-tricyclobutabenzene". Journal of the American Chemical Society. 98 (9): 2667. doi:10.1021/ja00425a046.
- ↑ Harruff, L. G.; Brown, M.; Boekelheide, V. (1978). "Hexaradialene: precursors and structure". Journal of the American Chemical Society. 100 (9): 2893. doi:10.1021/ja00477a055.
- ↑ Barkovich, A. J.; Strauss, E. S.; Vollhardt, K. P. C. (1977). "Hexaradialene". Journal of the American Chemical Society. 99 (25): 8321. doi:10.1021/ja00467a036.
- ↑ Schiess, P.; Heitzmann, M. (1978). "Hexakis (methylidene)-cyclohexane (?[6]Radialene?). Chemical and spectral properties". Helvetica Chimica Acta. 61 (2): 844. doi:10.1002/hlca.19780610232.
- ↑ Hopff, H.; Wick, A. K. (1961). "Zur Kenntnis der Hexaalkylbenzole. 2. Mitteilung. Seitenkettenhalogenierungen von Hexaäthylbenzol". Helvetica Chimica Acta. 44 (1): 19. doi:10.1002/hlca.19610440104.
- ↑ Iyoda, M.; Tanaka, S.; Otani, H.; Nose, M.; Oda, M. (1988). "A new approach to the construction of radialenes by the nickel-catalyzed cyclooligomerization of [3]cumulenes (butatrienes)". Journal of the American Chemical Society. 110 (25): 8494. doi:10.1021/ja00233a028.
- ↑ Stanger, A.; Ashkenazi, N.; Boese, R.; Bläser, D.; Stellberg, P. (1997). "Hexabromotricyclobutabenzene and Hexabromohexaradialene: Their Nickel-Mediated One-Pot Syntheses and Crystal Structure". Chemistry: A European Journal. 3 (2): 208. doi:10.1002/chem.19970030207.
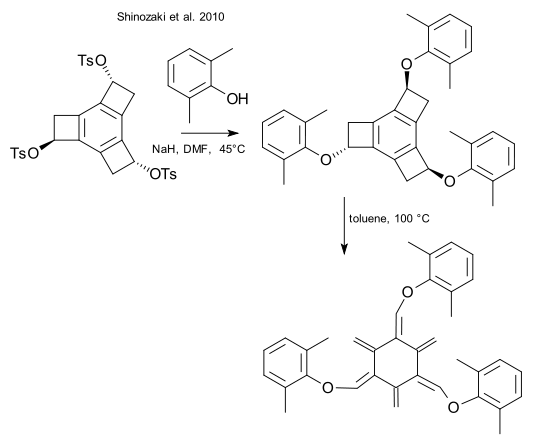
- ↑ Shinozaki, S.; Hamura, T.; Ibusuki, Y.; Fujii, K.; Uekusa, H.; Suzuki, K. (2010). "Hexaradialenes by Successive Ring Openings of Tris(alkoxy-tricyclobutabenzenes): Synthesis and Characterization". Angewandte Chemie International Edition in English. 49 (17): 3026–3029. PMID 20333636. doi:10.1002/anie.200907305.
- ↑ Synthesis and Diels–Alder Reactions of a Benzo[5]radialene Derivative Andreas A. von Richthofen, Liliana Marzorati, Lucas C. Ducati, and Claudio Di Vitta Organic Letters 2014 16 (15), 4020-4023 doi:10.1021/ol5018432
- ↑ Januszewski, J. A., Hampel, F., Neiss, C., Görling, A. and Tykwinski, R. R. (2014), Unexpected Formation of a [4]Radialene and Dendralenes by Addition of Tetracyanoethylene to a Tetraaryl[5]cumulene. Angew. Chem. Int. Ed., 53: 3743–3747. doi:10.1002/anie.201309355
- ↑ 2-D Coordination Polymers of Hexa(4-cyanophenyl)[3]-radialene and Silver(I): Anion···π-Interactions and Radialene C−H···Anion Hydrogen Bonds in the Solid-State Interactions of Hexaaryl[3]-radialenes with Anions Courtney A. Hollis, Lyall R. Hanton, Jonathan C. Morris, and Christopher J. Sumby Crystal Growth & Design 2009 9 (6), 2911-2916 doi:10.1021/cg9002302
- ↑ Two-Dimensional and Three-Dimensional Coordination Polymers of Hexakis(4-cyanophenyl)[3]radialene: The Role of Stoichiometry and Kinetics Courtney A. Hollis, Stuart R. Batten, and Christopher J. Sumby Crystal Growth & Design 2013 13 (6), 2350-2361 doi:10.1021/cg400036x