RU-28362
 | |
| Names | |
|---|---|
| IUPAC name
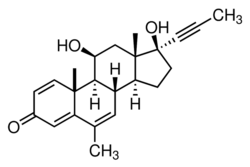
11β,17β-Dihydroxy-6-methyl-17α-(1-propynyl)androsta-1,4,6-trien-3-one | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| PubChem CID |
|
| |
| |
| Properties | |
| C23H28O3 | |
| Molar mass | 352.47 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
RU-28362 is a synthetic androstane glucocorticoid that was developed by Roussel Uclaf. It is a selective agonist of the glucocorticoid receptor (corticoid type II receptor), but not of the mineralocorticoid receptor (corticoid type I receptor).[1][2]
A similar compound is dexamethasone that also selectively binds to the glucocorticoid receptor with high affinity. This is in contrast to the natural steroid hormones cortisol or corticosterone, which bind to both of the corticosteroid receptors, though they bind to the mineralocorticoid receptor with greater affinity.[3]
See also
References
- ↑ Woolley C, Gould E, Sakai R, Spencer R, McEwen B (1991). "Effects of aldosterone or RU28362 treatment on adrenalectomy-induced cell death in the dentate gyrus of the adult rat". Brain Res. 554 (1–2): 312–5. PMID 1933312. doi:10.1016/0006-8993(91)90207-C.
- ↑ Wong D, Lesage A, Siddall B, Funder J (1992). "Glucocorticoid regulation of phenylethanolamine N-methyltransferase in vivo". FASEB J. 6 (14): 3310–5. PMID 1426768.
- ↑ Arriza, JL; Simerly, RB; Swanson, LW; Evans, RM (November 1988). "The neuronal mineralocorticoid receptor as a mediator of glucocorticoid response.". Neuron. 1 (9): 887–900. PMID 2856104. doi:10.1016/0896-6273(88)90136-5.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.