RNA virus
An RNA virus is a virus that has RNA (ribonucleic acid) as its genetic material.[1] This nucleic acid is usually single-stranded RNA (ssRNA) but may be double-stranded RNA (dsRNA).[2] Notable human diseases caused by RNA viruses include Ebola hemorrhoragic fever, SARS, the common cold, influenza, hepatitis C, West Nile fever, polio and measles.
The International Committee on Taxonomy of Viruses (ICTV) classifies RNA viruses as those that belong to Group III, Group IV or Group V of the Baltimore classification system of classifying viruses and does not consider viruses with DNA intermediates in their life cycle as RNA viruses.[3] Viruses with RNA as their genetic material but that include DNA intermediates in their replication cycle are called retroviruses, and comprise Group VI of the Baltimore classification. Notable human retroviruses include HIV-1 and HIV-2, the cause of the disease AIDS.
Another term for RNA viruses that explicitly excludes retroviruses is ribovirus.[4]
Characteristics
Single-stranded RNA viruses and RNA Sense
RNA viruses can be further classified according to the sense or polarity of their RNA into negative-sense and positive-sense, or ambisense RNA viruses. Positive-sense viral RNA is similar to mRNA and thus can be immediately translated by the host cell. Negative-sense viral RNA is complementary to mRNA and thus must be converted to positive-sense RNA by an RNA-dependent RNA polymerase before translation. As such, purified RNA of a positive-sense virus can directly cause infection though it may be less infectious than the whole virus particle. Purified RNA of a negative-sense virus is not infectious by itself as it needs to be transcribed into positive-sense RNA; each virion can be transcribed to several positive-sense RNAs. Ambisense RNA viruses resemble negative-sense RNA viruses, except they also translate genes from the positive strand.[5]
Double-stranded RNA viruses
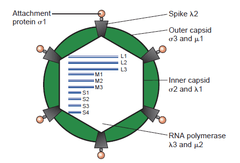
The double-stranded (ds)RNA viruses represent a diverse group of viruses that vary widely in host range (humans, animals, plants, fungi, and bacteria), genome segment number (one to twelve), and virion organization (Triangulation number, capsid layers, spikes, turrets, etc.). Members of this group include the rotaviruses, renowned globally as the most common cause of gastroenteritis in young children, and picobirnaviruses, renowned worldwide as the most commonly occurring virus in fecal samples of both humans and animals with or without signs of diarrhea. Bluetongue virus is an economically important pathogen of cattle and sheep. In recent years, remarkable progress has been made in determining, at atomic and subnanometeric levels, the structures of a number of key viral proteins and of the virion capsids of several dsRNA viruses, highlighting the significant parallels in the structure and replicative processes of many of these viruses.[2]
Mutation rates
RNA viruses generally have very high mutation rates compared to DNA viruses,[6] because viral RNA polymerases lack the proofreading ability of DNA polymerases.[7][8] This is one reason why it is difficult to make effective vaccines to prevent diseases caused by RNA viruses.[9] Retroviruses also have a high mutation rate even though their DNA intermediate integrates into the host genome (and is thus subject to host DNA proofreading once integrated), because errors during reverse transcription are embedded into both strands of DNA before integration.[10] Some genes of RNA virus are important to the viral replication cycles and mutations are not tolerated. For example, the region of the hepatitis C virus genome that encodes the core protein is highly conserved,[11] because it contains an RNA structure involved in an internal ribosome entry site.[12]
Replication
Animal RNA viruses are classified by the ICTV. There are three distinct groups of RNA viruses depending on their genome and mode of replication:
- Double-stranded RNA viruses (Group III) contain from one to a dozen different RNA molecules, each coding for one or more viral proteins.
- Positive-sense ssRNA viruses (Group IV) have their genome directly utilized as mRNA, with host ribosomes translating it into a single protein that is modified by host and viral proteins to form the various proteins needed for replication. One of these includes RNA-dependent RNA polymerase (RNA replicase), which copies the viral RNA to form a double-stranded replicative form. In turn this dsRNA directs the formation of new viral RNA.
- Negative-sense ssRNA viruses (Group V) must have their genome copied by an RNA replicase to form positive-sense RNA. This means that the virus must bring along with it the RNA replicase enzyme. The positive-sense RNA molecule then acts as viral mRNA, which is translated into proteins by the host ribosomes.
Retroviruses (Group VI) have a single-stranded RNA genome but, in general, are not considered RNA viruses because they use DNA intermediates to replicate. Reverse transcriptase, a viral enzyme that comes from the virus itself after it is uncoated, converts the viral RNA into a complementary strand of DNA, which is copied to produce a double-stranded molecule of viral DNA. After this DNA is integrated into the host genome using the viral enzyme integrase, expression of the encoded genes may lead to the formation of new virions.
Classification
Classification of the RNA viruses has proven to be a difficult problem. This is in part due to the high mutation rates these genomes undergo. Classification is based principally on the type of genome (double-stranded, negative- or positive-single-strand) and gene number and organisation. Currently there are 5 orders and 47 families of RNA viruses recognised. There are also many unassigned species and genera.
Related to but distinct from the RNA viruses are the viroids and the RNA satellite viruses. These are not currently classified as RNA viruses and are described on their own pages.
Positive strand RNA viruses
This is the single largest group of RNA viruses[13] with 30 families. Attempts have been made to group these families in higher orders. These proposals were based on an analysis of the RNA polymerases and are still under consideration. To date, the suggestions proposed have not been broadly accepted because of doubts over the suitability of a single gene to determine the taxonomy of the clade.
The proposed classification of positive-strand RNA viruses is based on the RNA-dependent RNA polymerase. Three groups have been recognised:[14]
I. Bymoviruses, comoviruses, nepoviruses, nodaviruses, picornaviruses, potyviruses, sobemoviruses and a subset of luteoviruses (beet western yellows virus and potato leafroll virus)—the picorna like group (Picornavirata).
II. Carmoviruses, dianthoviruses, flaviviruses, pestiviruses, statoviruses, tombusviruses, single-stranded RNA bacteriophages, hepatitis C virus and a subset of luteoviruses (barley yellow dwarf virus)—the flavi like group (Flavivirata).
III. Alphaviruses, carlaviruses, furoviruses, hordeiviruses, potexviruses, rubiviruses, tobraviruses, tricornaviruses, tymoviruses, apple chlorotic leaf spot virus, beet yellows virus and hepatitis E virus—the alpha like group (Rubivirata).
A division of the alpha-like (Sindbis-like) supergroup on the basis of a novel domain located near the N termini of the proteins involved in viral replication has been proposed.[15] The two groups proposed are: the 'altovirus' group (alphaviruses, furoviruses, hepatitis E virus, hordeiviruses, tobamoviruses, tobraviruses, tricornaviruses and probably rubiviruses); and the 'typovirus' group (apple chlorotic leaf spot virus, carlaviruses, potexviruses and tymoviruses).
The alpha like supergroup can be further divided into three clades: the rubi-like, tobamo-like, and tymo-like viruses.[16]
Additional work has identified five groups of positive-stranded RNA viruses containing four, three, three, three, and one order(s), respectively.[17] These fourteen orders contain 31 virus families (including 17 families of plant viruses) and 48 genera (including 30 genera of plant viruses). This analysis suggests that alphaviruses and flaviviruses can be separated into two families—the Togaviridae and Flaviridae, respectively—but suggests that other taxonomic assignments, such as the pestiviruses, hepatitis C virus, rubiviruses, hepatitis E virus, and arteriviruses, may be incorrect. The coronaviruses and toroviruses appear to be distinct families in distinct orders and not distinct genera of the same family as currently classified. The luteoviruses appear to be two families rather than one, and apple chlorotic leaf spot virus appears not to be a closterovirus but a new genus of the Potexviridae.
- Evolution
The evolution of the picornaviruses based on an analysis of their RNA polymerases and helicases appears to date to the divergence of the eukaryotes.[18] Their putative ancestors include the bacterial group II retroelements, the family of HtrA proteases and DNA bacteriophages.
Double-stranded RNA viruses
This analysis also suggests that the dsRNA viruses are not closely related to each other but instead belong to four additional classes—Birnaviridae, Cystoviridae, Partitiviridae, and Reoviridae — and one additional order (Totiviridae) of one of the classes of positive ssRNA viruses in the same subphylum as the positive-strand RNA viruses.
One study has suggested a that there are two large clades: One includes the Caliciviridae, Flaviviridae, and Picornaviridae families and a second that includes the Alphatetraviridae, Birnaviridae and Cystoviridae, Nodaviridae, and Permutotretraviridae families.[19]
Satellite viruses
A number of satellite viruses - viruses that require the assistance of another virus to complete their life cycle - are also known. Thei taxonomy has yet to be settled. The following four genera have been proposed for positive sense single stranded RNA satellite viruses that infect plants - Albetovirus, Aumaivirus, Papanivirus and Virtovirus.[20] A family - Sarthroviridae which includes the genus Macronovirus - has been proposed for the positive sense single stranded RNA satellite viruses that infect arthropods.
Group III—dsRNA viruses
There are twelve families and a number of unassigned genera and species recognised in this group.[7]
- Family Amalgaviridae
- Family Birnaviridae
- Family Chrysoviridae
- Family Cystoviridae
- Family Endornaviridae
- Family Hypoviridae
- Family Megabirnaviridae
- Family Partitiviridae
- Family Picobirnaviridae
- Family Reoviridae—includes Rotavirus
- Family Totiviridae
- Family Quadriviridae
- Genus Botybirnavirus
- Unassigned species
- Botrytis porri RNA virus 1
- Circulifer tenellus virus 1
- Colletotrichum camelliae filamentous virus 1
- Cucurbit yellows associated virus
- Sclerotinia sclerotiorum debilitation-associated virus
- Spissistilus festinus virus 1
Group IV—positive-sense ssRNA viruses
There are three orders and 34 families recognised in this group. In addition, there are a number of unclassified species and genera.
- Order Nidovirales
- Family Arteriviridae
- Family Coronaviridae — includes Coronavirus, SARS
- Family Mesoniviridae
- Family Roniviridae
- Order Picornavirales
- Family Dicistroviridae
- Family Iflaviridae
- Family Marnaviridae
- Family Picornaviridae—includes Poliovirus, Rhinovirus (a common cold virus), Hepatitis A virus
- Family Secoviridae includes subfamily Comovirinae
- Genus Bacillariornavirus
- Genus Dicipivirus
- Genus Labyrnavirus
- Genus Sequiviridae
- Species Kelp fly virus
- Order Tymovirales
- Family Alphaflexiviridae
- Family Betaflexiviridae
- Family Gammaflexiviridae
- Family Tymoviridae
- Unassigned
- Family Alphatetraviridae
- Family Alvernaviridae
- Family Astroviridae
- Family Barnaviridae
- Family Benyviridae
- Family Bromoviridae
- Family Caliciviridae — includes Norwalk virus
- Family Carmotetraviridae
- Family Closteroviridae
- Family Flaviviridae — includes Yellow fever virus, West Nile virus, Hepatitis C virus, Dengue fever virus, Zika virus
- Family Fusariviridae
- Family Hepeviridae
- Family Leviviridae
- Family Luteoviridae — includes Barley yellow dwarf virus
- Family Narnaviridae
- Family Nodaviridae
- Family Permutotetraviridae
- Family Potyviridae
- Family Statovirus
- Family Togaviridae — includes Rubella virus, Ross River virus, Sindbis virus, Chikungunya virus
- Family Tombusviridae
- Family Virgaviridae[21]
- Unassigned genera
- Genus Blunervirus
- Genus Cilevirus
- Genus Higrevirus
- Genus Idaeovirus
- Genus Negevirus
- Genus Ourmiavirus
- Genus Polemovirus
- Genus Sinaivirus
- Genus Sobemovirus
- Unassigned species
- Acyrthosiphon pisum virus
- Bastrovirus
- Blackford virus
- Blueberry necrotic ring blotch virus
- Chara australis virus
- Extra small virus
- Jingmen tick virus
- Le Blanc virus
- Nesidiocoris tenuis virus 1
- Nylanderia fulva virus 1
- Orsay virus
- Plasmopara halstedii virus
- Rosellinia necatrix fusarivirus 1
- Santeuil virus
- Solenopsis invicta virus 2
- Solenopsis invicta virus 3
Satellite viruses
- Family Sarthroviridae
- Genus Albetovirus
- Genus Aumaivirus
- Genus Papanivirus
- Genus Virtovirus
- Chronic bee paralysis virus
Group V—negative-sense ssRNA viruses
Two orders and twenty one families are currently recognised in this group. Several of these families contain extremely similar genetic structures and are classified under the order Mononegavirales.[22] A number of unassigned species and genera are yet to be classified.[7]
- Order Mononegavirales
- Family Bornaviridae - includes Borna disease virus
- Family Filoviridae - includes Ebola virus, Marburg virus
- Family Mymonaviridae
- Family Nyamiviridae
- Family Paramyxoviridae - includes Measles virus, Mumps virus, Nipah virus, Hendra virus, and Newcastle disease virus
- Family Pneumoviridae - includes Human respiratory syncytial virus
- Family Rhabdoviridae - includes Rabies virus
- Family Sunviridae
- Genus Anphevirus
- Genus Arlivirus
- Genus Chengtivirus
- Genus Crustavirus
- Genus Wastrivirus
- Order Bunyavirales
- Family Feraviridae
- Family Fimoviridae
- Family Hantaviridae
- Family Jonviridae
- Family Nairoviridae
- Family Peribunyaviridae
- Family Phasmaviridae
- Family Phenuiviridae
- Family Tospoviridae
- Unassigned families:
- Family Arenaviridae — includes Lassa virus
- Family Ophioviridae
- Family Orthomyxoviridae — includes Influenza viruses
- Unassigned genera:
- Genus Deltavirus — includes Hepatitis D virus
- Unassigned species:
- Taastrup virus
Gallery
Notes
The majority of fungal viruses are double-stranded RNA viruses. A small number of positive-strand RNA viruses have been described. One report has suggested the possibility of a negative stranded virus.[23]
See also
- Virus classification
- List of viruses
- Viral replication
- Positive/negative-sense
- Animal viruses
- Double-stranded RNA viruses
- Retrovirus
- DNA viruses
- Norovirus cis-acting replication element
- Viroid
References
- ↑ MeSH, retrieved on 12 April 2008.
- ↑ "Listing in Taxonomic Order—Index to ICTV Species Lists". Retrieved 2008-04-11.
- ↑ Drake JW, Holland JJ (November 1999). "Mutation rates among RNA viruses". Proc. Natl. Acad. Sci. U.S.A. 96 (24): 13910–3. PMC 24164
 . PMID 10570172. doi:10.1073/pnas.96.24.13910.
. PMID 10570172. doi:10.1073/pnas.96.24.13910. - ↑ Nguyen M, Haenni AL (2003). "Expression strategies of ambisense viruses". Virus Res. 93 (2): 141–50. PMID 12782362. doi:10.1016/S0168-1702(03)00094-7.
- ↑ Sanjuan, R.; Nebot, M. R.; Chirico, N.; Mansky, L. M.; Belshaw, R. (2010). "Viral Mutation Rates" (PDF). Journal of Virology. 84 (19): 9733–9748. ISSN 0022-538X. doi:10.1128/JVI.00694-10.
- 1 2 3 Klein, Donald W.; Prescott, Lansing M.; Harley, John (1993). Microbiology. Dubuque, Iowa: Wm. C. Brown. ISBN 0-697-01372-3.
- ↑ Martinez MA, et al. (2012). "Quasispecies Dynamics of RNA Viruses". In Witzany, G. Viruses: Essential Agents of Life. Springer. pp. 21–42. ISBN 978-94-007-4898-9.
- ↑ Steinhauer DA, Holland JJ (1987). "Rapid evolution of RNA viruses". Annu. Rev. Microbiol. 41: 409–33. PMID 3318675. doi:10.1146/annurev.mi.41.100187.002205.
- ↑ Boutwell CL, Rolland MM, Herbeck JT, Mullins JI, Allen TM (October 2010). "Viral Evolution and Escape during Acute HIV-1 Infection". J. Infect. Dis. 202 (Suppl 2): S309–14. PMC 2945609
 . PMID 20846038. doi:10.1086/655653.
. PMID 20846038. doi:10.1086/655653. - ↑ Bukh J, Purcell RH, Miller RH (August 1994). "Sequence analysis of the core gene of 14 hepatitis C virus genotypes". Proc. Natl. Acad. Sci. U.S.A. 91 (17): 8239–43. PMC 44581
 . PMID 8058787. doi:10.1073/pnas.91.17.8239.
. PMID 8058787. doi:10.1073/pnas.91.17.8239. - ↑ Tuplin A, Evans DJ, Simmonds P (October 2004). "Detailed mapping of RNA secondary structures in core and NS5B-encoding region sequences of hepatitis C virus by RNase cleavage and novel bioinformatic prediction methods". J. Gen. Virol. 85 (Pt 10): 3037–47. PMID 15448367. doi:10.1099/vir.0.80141-0.
- ↑ Francki, R.I.B; Fauquet, C. M.; Knudson, D. L.; Brown, F. (1991). Classification and nomenclature of viruses. Fifth report of the International Committee on Taxonomy of Viruses, Archives of Virology (Suppl. 2). ISBN 978-3-7091-9163-7.
- ↑ Koonin EV (September 1991). "The phylogeny of RNA-dependent RNA polymerases of positive-strand RNA viruses". J. Gen. Virol. 72 (Pt 9): 2197–206. PMID 1895057. doi:10.1099/0022-1317-72-9-2197.
- ↑ Rozanov MN, Koonin EV, Gorbalenya AE (August 1992). "Conservation of the putative methyltransferase domain: a hallmark of the 'Sindbis-like' supergroup of positive-strand RNA viruses". J. Gen. Virol. 73 (Pt 8): 2129–34. PMID 1645151. doi:10.1099/0022-1317-73-8-2129.
- ↑ Koonin EV, Dolja VV (1993). "Evolution and taxonomy of positive-strand RNA viruses: implications of comparative analysis of amino acid sequences". Crit. Rev. Biochem. Mol. Biol. 28 (5): 375–430. PMID 8269709. doi:10.3109/10409239309078440.
- ↑ Ward CW (1993). "Progress towards a higher taxonomy of viruses". Res Virol. 144 (6): 419–453. PMID 8140287. doi:10.1016/S0923-2516(06)80059-2.
- ↑ Koonin EV, Wolf YI, Nagasaki K, Dolja VV (December 2008). "The Big Bang of picorna-like virus evolution antedates the radiation of eukaryotic supergroups". Nat. Rev. Microbiol. 6 (12): 925–39. PMID 18997823. doi:10.1038/nrmicro2030.
- ↑ Gibrat JF, Mariadassou M, Boudinot P, Delmas B (2013). "Analyses of the radiation of birnaviruses from diverse host phyla and of their evolutionary affinities with other double-stranded RNA and positive strand RNA viruses using robust structure-based multiple sequence alignments and advanced phylogenetic methods". BMC Evol. Biol. 13: 154. PMC 3724706
 . PMID 23865988. doi:10.1186/1471-2148-13-154.
. PMID 23865988. doi:10.1186/1471-2148-13-154. - ↑ Krupovic M, Kuhn JH, Fischer MG (2016) A classification system for virophages and satellite viruses. Arch Virol 161(1):233-247 doi: 10.1007/s00705-015-2622-9
- ↑ Adams MJ, Antoniw JF, Kreuze J (2009). "Virgaviridae: a new family of rod-shaped plant viruses". Arch Virol. 154 (12): 1967–72. PMID 19862474. doi:10.1007/s00705-009-0506-6.
- ↑ Cann, Alan (2011). Principles of Molecular Virology. Academic Press. ISBN 978-0-12-384939-7.
- ↑ Kondo, H.; Chiba, S.; Toyoda, K.; Suzuki, N. (2012). "Evidence for negative-strand RNA virus infection in fungi". Virology. 435 (2): 201–9. PMID 23099204. doi:10.1016/j.virol.2012.10.002.
External links
- RNA Viruses at the US National Library of Medicine Medical Subject Headings (MeSH)
- Animal viruses




_EM_PHIL_2175_lores.jpg)

_EM_18_lores.jpg)